Rank the following organic molecules in order of increasing solubility in water: CI- - (b) (c) (a)
Question:
Rank the following organic molecules in order of increasing solubility in water:
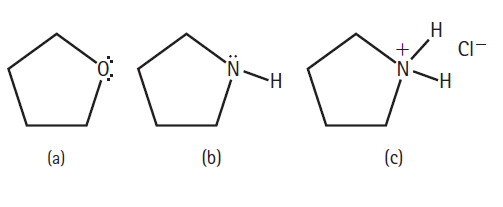
Transcribed Image Text:
Н CI- -н (b) (c) (a)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (12 reviews)
Compound a is an ether which has limited solubility in wate...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Conceptual Physical Science
ISBN: 978-0134060491
6th edition
Authors: Paul G. Hewitt, John A. Suchocki, Leslie A. Hewitt
Question Posted:
Students also viewed these Physics questions
-
Rank the following compounds in order of increasing solubility in water: CH 3 CH 2 OH Ethanol CH 3 CH 2 CH 2 CH 2 OH Butanol CH 3 CH 2 CH 2 CH 2 CH 2 CH 2 OH Hexanol
-
Rank each of the following sets of molecules in order of increasing SN2 reactivity. (a) CH3CH2Br, CH3Br, (CH3)2CHBr (b) (CH3)2CHCH2CH2Cl, (CH3)2CHCH2Cl, (CH3)2CHCl (c) (d) CH,CH,CI, CH,CH2I. CI...
-
Rank the following molecules in order of increasing boiling point (without looking up the real values!): (a) 3-methylheptane; (b) Octane; (c) 2,4-dimethylhexane; (d) 2,2,4-trimethylpentane.
-
Troy Engines, Ltd., manufactures a variety of engines for use in heavy equipment. The company has always produced all of the necessary parts for its engines, including all of the carburetors. An...
-
Jingie owns two parcels of business land ( 1231 assets). One parcel can be sold at a loss of $60,000, and the other parcel can be sold at a gain of $70,000. Jingie has no nonrecaptured 1231 losses...
-
The beam is constructed from two boards fastened together at the top and bottom with three rows of nails spaced every 4 in. If each nail can support a 400-lb shear force, determine the maximum shear...
-
E 7-4 Subsidiary purchases parent bonds On January 1, 2014, Petr SA purchased half of Lenka SAs outstanding 10 percent bond for $550,000 cash. Petr SA was the 80 percent-owned subsidiary of Lenka SA...
-
On July 1, 2015, Ashlock Chemical Company issued $4,000,000, 10%, 10 year bonds at $4,543,627. This price resulted in an 8% effective-interest rate on the bonds. Ashlock uses the effective-interest...
-
You are the vice president of finance of Concord Corporation, a retail company that prepared two different schedules of gross margin for the first quarter ended March 31, 2020. These schedules appear...
-
iCover produces bags for carrying laptop computers. iCover sells 1,000,000 units each year at a price of $20 per unit and a contribution margin of 40%. To respond to customer complaints, iCover's...
-
Rank the following organic molecules in order of increasing solubility in water: (b) (c) (a)
-
What property of carbon allows for the formation of so many different organic molecules?
-
The key to a project financing is to finance a project with as little recourse to the sponsor as possible. Explain why.
-
(25 Points) University Painting is considering investing in a new paint sprayer to allow them to paint more classrooms in less time. The sprayer would have the following cash flow and cost of capital...
-
Use the following information for questions 1 and 2. Caterpillar Financial Services Corp. (a subsidiary of Caterpillar) and Sterling Construction sign a lease agreement dated January 1, 2020, that...
-
Summit Regional Medical Center operates as a private not-for-profit hospital, providing services to a community of 20,000 and the surrounding rural areas. Summit has maintained a banking relationship...
-
Woodland Wearables produces two models of a smart watch, the Basic and the Flash. The watches have the following characteristics: Basic Flash Selling price per watch $ 3 3 0 $ 4 9 0 Variable cost per...
-
Introduction This practice case has been designed to give introductory-level business students practical experience in the application of accounting concepts. This practice case will provide students...
-
Which astronomical objects that appear as points of light to the unaided eye appear as disks with the help of a telescope?
-
Which of the following is NOT a magnetic dipole when viewed from far away? a) A permanent bar magnet. b) Several circular loops of wire closely stacked together with the same current running in each...
-
Show that, for the case of natural convection adjacent to a plane vertical wall, the appropriate integral equations for the hydrodynamic and thermal boundary layers are and vz(T T)dy dx ly=0 dvx ...
-
Ammonia (NH 3 ) and hydrogen sulfide (H 2 S) must both be stripped from wastewater in a packed tower before the wastewater can be treated for reuse. Individual mass-transfer coefficients for ammonia...
-
Using the integral relations from Problem 19.17, and assuming the velocity and temperature profiles of the form and where δ is the thickness of both the hydrodynamic and thermal boundary...
-
Due to the relationship of financial statements, the statement of stockholders' equity links the income statement to the balance sheet. True or False?
-
Troy Engines, Limited, manufactures a variety of engines for use in heavy equipment. The company has always produced all of the necessary parts for its engines, including all of the carburetors. An...
-
Trey is single and has no qualifying child. His adjusted gross income is $12,355. In order to claim the Earned Income Tax Credit, he must meet which of the following requirements? He cannot be the...
Financial Risk Management Models History And Institutions 1st Edition - ISBN: 1118022912 - Free Book

Study smarter with the SolutionInn App


