There is an excess of at least one of the reactant molecules. Which one? B
Question:
There is an excess of at least one of the reactant molecules. Which one?
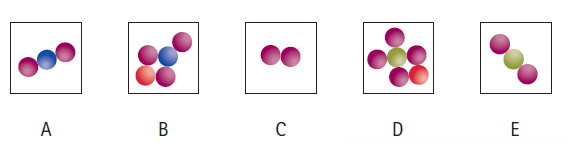
Transcribed Image Text:
B
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 87% (16 reviews)
There is one diatomic molecule that ...View the full answer

Answered By

Benish Ahmad
I'm a professional software engineer. I'm lectutrer at GCUF and I have 3 years of teaching experience. I'm looking forward to getting mostly computer science work including:
Programming fundamentals
Object oriented programming
Data structures
object oriented design and analysis
Database system
Computer networks
Discrete mathematics
Web application
I am expert in different computer languages such as C++, java, JavaScript, Sql, CSS, Python and C#. I'm also have excellent knowledge of essay writing and research. I have worked in other Freelancing website such as Fiverr and Upwork. Now I have finally decided to join the SolutionInn platform to continue with my explicit work of helping dear clients and students to achieve their academic dreams. I deliver plagiarism free work and exceptional projects on time. I am capable of working under high pressure.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Conceptual Physical Science
ISBN: 978-0134060491
6th edition
Authors: Paul G. Hewitt, John A. Suchocki, Leslie A. Hewitt
Question Posted:
Students also viewed these Physics questions
-
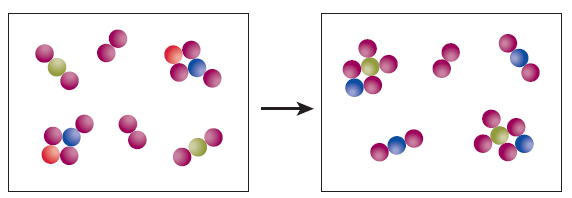
Assume the illustrations above are two frames of a movieone from before the reaction and the other from after the reaction. How many diatomic molecules are represented in this movie?
-
Use the bond energies in Table 17.1 and the accounting format shown in Section 17.5 to determine whether these reactions are exothermic or endothermic: a. b. Table 17.1 N-N - + +N, 0+- +
-
When a chemical loses a hydrogen ion, is it behaving as an acid or a base?
-
In the first case, the relationship between F and H is found to be inverse when the height decrease the area will increase, and the force will increase too. Therefore, I need a logical and practical...
-
The following transactions pertain to 2014, the first year of operations of the Barlett Company. All inventory was started and completed during 2014. Assume that all transactions are cash...
-
Draw up an income statement and balance sheet for this company for 2016 and 2017. Use the following information for Ingersoll, Inc., (assume the tax rate is 35 percent): 2016 2017 $ 40,743 $ 43,277...
-
Grasp how the study of consumers helps fashion brands and businesses to improve their marketing strategies. LO.1
-
Montel Companys July sales budget calls for sales of $ 600,000. The store expects to begin July with $ 50,000 of inventory and to end the month with $ 40,000 of inventory. Gross margin is typically...
-
Security A: E(r) = .15; standard deviation = ..2000 Security B: E(r) = .10; standard deviation = .1500 Security C: E(r) = .12; standard deviation = .3160 Security D: E(r) = .13; standard deviation =...
-
Jones Inc. is preparing an aggregate production plan for next year. The company expects demand to be 1,000 units in quarter 1; 2,000 units in quarter 2; 4,000 units in quarter 3; and 3,000 units in...
-
Which equation best describes this reaction? (a) 2 AB 2 + 2 DCB 3 + B 2 2 DBA 4 + 2 CA 2 (b) 2 AB 2 + 2 CDA 3 + B 2 2 C2A 4 + 2 DBA (c) 2 AB 2 + 2 CDA 3 + A 2 2 DBA 4 + 2 CA 2 (d) 2 BA 2 + 2 DCA 3...
-
Balance these equations: (a) ____ Fe(s) + ____ S(s) ____ Fe 2 S 3 (s) (b) ____ P 4 (s) + ____ H 2 ( g ) ____ PH 3 ( g) (c) ____ NO( g ) + ____ Cl 2 ( g) ____ NOCI( g) (d) ____ SiCl 4 (l ) + ____...
-
Design an efficient riser system for the casting shown in Figure 9-31. Be sure to include a sketch of the system, along with appropriate dimensions. 2 2 2 8 13 2 3 4 12
-
1. Why do companies that choose to open subsidiaries in other countries have different HR responsibilities? 2. How has globalization allowed companies to become "global companies" more easily? 3....
-
Is Kroger's innovation Product-related or process-related? Do the innovations tend to be incremental or radical? https://www.thekrogerco.com/about-kroger/our-business/ Kroger Co. opens new spoke in...
-
Define what is Process Mapping/Value Stream Mapping How do you apply process mapping methodology? What are the advantages of leaders using process mapping Identify a real world business...
-
What role do formalized processes and protocols play in highly structured organizations, and how can organizations balance the need for structure with the imperative for flexibility and innovation ?
-
In what ways do decision-makers balance quantitative data with qualitative insights to optimize complex strategic choices, especially in high-stakes business environments where traditional metrics...
-
Determine whether the statement is true or false. If it is true, explain why. If it is false, explain why or give an example that disproves the statement. The equation y' = x + y is separable.
-
Fahrad Inc. sells all of its product on account. Fahrad has the following accounts receivable payment experience: Percent paid in the month of sale .........10 Percent paid in the month after the...
-
A helium balloon hovers in midair, neither ascending nor descending. Is it in equilibrium? What forces act on it?
-
When you fly in an airplane at night in smooth air, you have no sensation of motion, even though the plane may be moving at 800 km/h (500 mi/h). Why?
-
If the two ends of a rope in equilibrium are pulled with forces of equal magnitude and opposite directions, why isnt the total tension in the rope zero?
-
Prepare journal entries to record the following events: Jul. 1 Klemens Company accepted a 5%, 3-month, $8,000 note dated July 1 from Mox Company for the balance due on Mox's account. Jul. 31 Klemens...
-
FINANCIAL STATEMENT ANALYSIS INSTRUCTIONS 1. PREPARE RATIO ANALYSIS REPORT ( word file) Format 1. Introduction 2. Importance of Financial Statements 3. Importance of Financial statement analysis and...
-
Let us assume that Europe is in recession, China's economy is slowing down, and the US economy is growing at 1-2%. Use these assumptions to invest in 4 ETFs (electronically traded funds). The 4 ETFs...

Study smarter with the SolutionInn App


