Question: Two carbon atoms connected by a single bond can rotate relative to each other. This ability to rotate can give rise to numerous conformations (spatial
Two carbon atoms connected by a single bond can rotate relative to each other. This ability to rotate can give rise to numerous conformations (spatial orientations) of an organic molecule. Is it also possible for two carbon atoms connected by a double bond to rotate relative to each other? Hold two toothpicks side by side and attach one jellybean to each end such that each jellybean has both toothpicks poked into it. Hold one jellybean while rotating the other. What kind of rotations are possible? Relate what you observe to the carbon–carbon double bond. Which structure of 19.7 do you suppose has more possible conformations: butane or 2-butene? What do you suppose is generally true about the ability of atoms connected by a carbon–carbon triple bond to twist relative to each other?

Figure 19.7
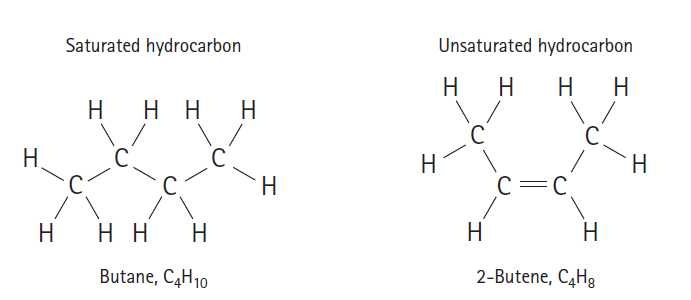
Saturated hydrocarbon Unsaturated hydrocarbon Butane, C4H10 2-Butene, C4H3
Step by Step Solution
3.36 Rating (162 Votes )
There are 3 Steps involved in it
Two carbons connected by a double bond are n... View full answer

Get step-by-step solutions from verified subject matter experts


