Use the bond energies in Table 17.1 and the accounting format shown in Section 17.5 to determine
Question:
Use the bond energies in Table 17.1 and the accounting format shown in Section 17.5 to determine whether these reactions are exothermic or endothermic:
H2 + Cl2 ¡ 2 HCl
2 HC‚CH + 5 O2 → 4 CO2 + 2 H2O
Table 17.1
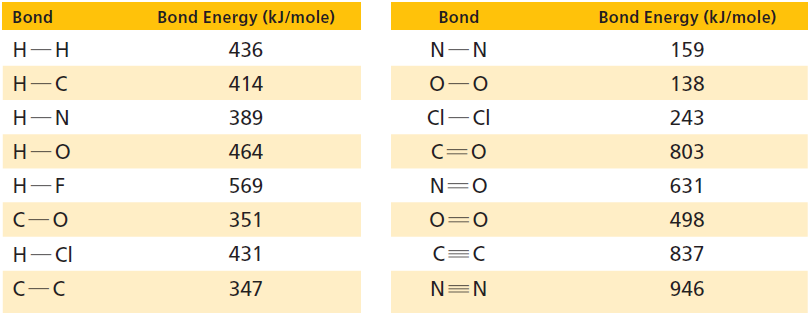
Transcribed Image Text:
Bond Energy (kJ/mole) Bond Energy (kJ/mole) Bond Bond Н-Н 436 159 Н-С 414 138 Cl-CI 389 Н—N 243 803 Н—О 464 C=0 Н-—F 569 N=0 631 351 498 Н— СІ 431 837 N=N 347 946
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)
a Energy to break bonds Energy released from bond formation H x H 43...View the full answer

Answered By

Amos Kiprotich
I am a wild researcher and I guarantee you a well written paper that is plagiarism free. I am a good time manager and hence you are assured that your paper will always be delivered a head of time. My services are cheap and the prices include a series of revisions, free referencing and formatting.
4.90+
15+ Reviews
21+ Question Solved
Related Book For 

Conceptual Physical Science
ISBN: 978-0134060491
6th edition
Authors: Paul G. Hewitt, John A. Suchocki, Leslie A. Hewitt
Question Posted:
Students also viewed these Physics questions
-
Use the average bond energies in Table 4.3 to estimate ÎU for the reaction C 2 H 4 (g) + H 2 (g) C 2 H 6 (g). Also calculate ÎU o R from the tabulated values of ÎH o f for reactant...
-
The following table summarizes 204 endothermic reactions involving sodium bicarbonate. Let A denote the event that a reactions final temperature is 271 K or less. Let B denote the event that the heat...
-
2 Section 3 Date 4567 8 9 10 11 12 13 14 15 16 STRANK 21 22 23 Pharoah Inc. sells storage buildings of various sizes to homeowners and businesses. Respond to the requirements related to the following...
-
apple company: 1.what are key characteristics of the industry? 2. where is the company in its life cycle?
-
Arthur Wesson, an unmarried individual who is age 68, reports 2014 taxable income of $160,000. He records AMT positive adjustments of $40,000 and tax preferences of $35,000. a. What is Arthur's AMT?...
-
The shaft is supported by a smooth thrust bearing at A and smooth journal bearing at C. If d = 3 in., determine the absolute maximum bending stress in the shaft. -3 ft -3 ft -3 ft- 1800 lb 3600 Ib
-
2. Son sold equipment to Pop for $50,000 on January 1, 2015, at which time the equipment had a book value of $20,000 and a five-year remaining useful life (included in plant assets in the financial...
-
Dr. Miriam Johnson has been teaching accounting for over 20 years. From her experience she knows that 60% of her students do homework regularly. Moreover, 95% of the students who do their homework...
-
You have the opportunity to bid on a project that involves manufacturing 110,000 units per year for 4 years. The project will require $698,000 in fixed assets that would be depreciated straight-line...
-
Lee Werner is general manager of Worldwide Salons. During 2024, Werner worked for the company all year at a $12,400 monthly salary. He also earned a year-end bonus equal to 20% of his annual salary....
-
Rank the following in order of increasing number of atoms: (a) 52 g of vanadium, V; (b) 52 g of chromium, Cr; (c) 52 g of manganese, Mn.
-
Bakers yeast contains a biological catalyst known as catalase, which catalyzes the transformation of hydrogen peroxide, H 2 O 2 , into oxygen, O 2 , and water, H 2 O. Write a balanced equation for...
-
Consider a block of pure aluminum measuring 25 300 200mm 3 . The density and specific heat of aluminium are approximately 2702 kg/m 3 and 903 J/kg K, respectively. Estimate the change in internal...
-
As a new principal, I assigned a teacher to a different grade for the coming year. I did not expect to cause the anxiety it did. The teacher first came to me in tears and begged for her assignment to...
-
Peruse the following websites to learn about the different ways of categorizing leadership. 1. https://www.businessnewsdaily.com/9789-leadership-types.html 2....
-
Making Consumer Choices The Espresso Machine (25 points) In real life, you must often make choices about whether to buy something pre-made or make it yourself. There are many things to consider:...
-
1) Read over the article/case and summarize what it is referring to in your own words. 2) What type of leadership traits can you describe in the case study? Use materials both from the handout and...
-
After reading or watching, https://smallbusiness.chron.com/internal-analysis-important-80513.html https://www.indeed.com/career-advice/career-development/internal-analysis...
-
During what divisions of geologic time have living things existed on the earth?
-
Suppose that a company has 10.000 outstanding shares in the beginning of the year. On April 1st, the company increases its shares by 6.000. On July 1st, the company increases its shares again, but...
-
Liquid nitrogen at 77 K is stored in a cylindrical container having an inside diameter of 25 cm. The cylinder is made of stainless steel and has a wall thickness of 1.2 cm. Insulation is to be added...
-
The Fourier field equation in cylindrical coordinates is a. What form does this equation reduce to for the case of steady-state, radial heat transfer? b. Given the boundary conditions T = T i at r =...
-
Perform the same operations as in parts (a), (b), and (c) of Problem 16.1 with respect to a spherical system. Data From Problem 16.1 The Fourier field equation in cylindrical coordinates is a. What...
-
The payroll register of Ruggerio Co. indicates $13,800 of social security withheld and $3,450 of Medicare tax withheld on total salaries of $230,000 for the period. Federal withholding for the period...
-
All of the following are included on Form 1040, page 1, EXCEPT: The determination of filing status. The Presidential Election Campaign check box. The income section. The paid preparer signature line.
-
Question One: (25 marks) (X) Inc. purchased 80% of the outstanding voting shares of (Y) for $360,000 on July 1, 2017. On that date, (Y) had common shares and retained earnings worth $180,000 and...

Study smarter with the SolutionInn App


