Use the average bond energies in Table 4.3 to estimate ÎU for the reaction C 2 H
Question:
Table 4.3
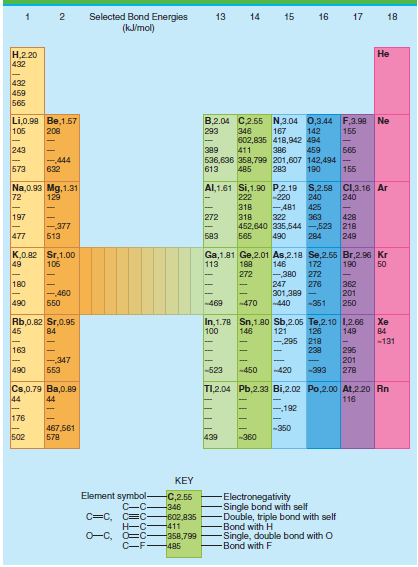
Transcribed Image Text:
Selected Bond Energies (klmol) 13 14 15 16 17 18 Н.220 432 Не 432 459 565 B,2.04 C2.55 N,3.04 0,3.44 F,3.98 Ne Li,0.90 Be,1.57 105 208 293 346 167 142 155 602,835 418,942 494 243 Б65 389 411 386 459 -444 536,636 358,799 201,607 142,494 155 573 632 613 485 283 190 Al,1.61 Si,1.90 P.2.19 S,2.58 C,3.16 Ar Na,0.93 Mg, 1.31 72 129 222 -220 240 240 -481 322 363 452,640 335,544 -523 218 318 425 197 272 318 428 -377 477 583 249 513 565 490 284 K,0.82 Sr,1.00 49 Ga,1.81 Ge,2.01 As,2.18 Se,2.55 Br,2.96 Kr 146 106 113 188 172 190 50 272 -380 272 180 247 362 201 250 276 301,389 - -440 -460 490 Б50 -469 470 -351 In,1.78 Sn,1.80 Sb,2.05 Te,2.10 1,2.66 121 -295 Rb,0.82 Sr,0.95 45 Xe 100 149 84 146 126 84 218 238 -131 163 296 201 278 -,347 553 490 -523 -420 450 -393 TI,2.04 Pb,2.33 Bi,2.02 Po,2.00o At,220 Rn Cs,0.79 Ba,0.89 44 116 44 192 176 -350 467,561 578 502 439 -360 KEY Element symbol- C-C C=C, C=C Electronegativity Single bond with self Double, triple bond with self Bond with H Single, double bond with O -Bond with F C,2.55 -346 602,835 -411 O-C, O=C- -358,799 C-F485
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
U R C C bond energy 6C H bond energy H H bond energy C C bond energy 4C H bond energy U R 346 kJ mo...View the full answer

Answered By

Nimlord Kingori
2023 is my 7th year in academic writing, I have grown to be that tutor who will help raise your grade and better your GPA. At a fraction of the cost on other sites, I will work on your assignment by taking it as mine. I give it all the attention it deserves and ensures you get the grade that I promise. I am well versed in business-related subjects, information technology, Nursing, history, poetry, and statistics. Some software's that I have access to are SPSS and NVIVO. I kindly encourage you to try me; I may be all that you have been seeking, thank you.
4.90+
360+ Reviews
1070+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Basing your answers on the bond dissociation energies in Table 4.3, calculate which of the following reactions are endothermic and which are exothermic: (a) (CH3)2CHOH + HF - (CH3)2CHF + H2O (b)...
-
Use the following data (in kJ/ mol) to estimate ÎH for the reaction S-(g) + e- S2-(g). Include an estimate of uncertainty. S(s) S(g) ÎH = 277 kJ/ mol S(g) + e- S-(g) ÎH = 200. kJ/...
-
The rates of many atmospheric reactions are accelerated by the absorption of light by one of the reactants. For example, consider the reaction between methane and chlorine to produce methyl chloride...
-
A jet is traveling westward with the sun directly overhead (the jet is on a line between the sun and the center of the Earth). How fast must the jet fly in order to keep the sun directly overhead?...
-
Which of the following statements is NOT CORRECT? (a) When a corporation's shares are owned by a few individuals who own most of the stock or are part of the firm's management, we say that the firm...
-
The velocity v (in ft/s) of a projectile launched upward from the ground is given by v = 32t + 56, where t is given in seconds. Given that speed = |velocity|, find the times at which the speed is...
-
14. What are the major revenue groupings of hospitals? Give an example of a revenue item that would be included in each grouping.
-
Calgary Injection Moulding operates a job-order costing system and applies overhead cost to jobs on the basis of machine-hours. In computing an overhead rate for the year, the company's estimates...
-
derson Corporation has found that 60% of its sales in any given month are credit tales, while the remainderare cash les of the credits, Anderson Corporation has experienced the fofowing colecion...
-
Using the Blazer Corporation information and financial statements created in unit 2, create a cash flow statement for 2018. Then answer the following questions about the statement and the...
-
Using the protein DSC data in Problem P4.10, calculate the enthalpy change between the T = 288 K and T = 318 K. Give your answer in units of kJ per mole. Assume the molecular weight of the protein is...
-
In the compounds below, classify each bond as covalent, polar covalent, or ionic: a) NaBr b) NaOH c) NaOCH 3 d) CH 3 OH e) CH 2 O
-
I spend half my time trying to figure out these accounting reports, said Paul Hribar in total frustration. He was trying to figure out whether to introduce a new product and was referring to the...
-
The table below shows the population of Mozambique between 1960 and 2010. This data can be modelled using an exponential function of the form P = ab t , where t is the time in years since 1960 and a...
-
Southco is a medium-sized American-owned global manufacturer of access hardware solutions, such as latches and hinges, used for applications in the aircraft, railway, computer and automotive...
-
To what extent do staffing processes at the Dionysos reflect the strategic approach to recruitment and selection encapsulated by the conceptual framework and model depicted in Key Concepts 8.4 and...
-
Im an accounting major, not an operations expert, yelled just-promoted Bob Barthrow, the executive vice president of the Midwest Frequent Flyer Call Center, during a senior-level management meeting....
-
The Hudson Jewelers case study can be found in Appendix C. Chapter 14 Case Question for Discussion: 1.Customer demand (weekly visits) at Hudson Jewelers is highly seasonal, as shown in the worksheet...
-
In Exercises 5970, the domain of each piecewise function is (- , ).a. Graph each function.b. Use your graph to determine the functions range. if if x if 0 f(x) = -X 42 x < -4 -4 x < 0 x 0
-
What is the difference between adsorption and absorption?
-
Derive an expression for the rate of disappearance of a species A in a photochemical reaction for which the mechanism is: Hence, show that rate measurements will give only a combination of k2 and k3...
-
Photolysis of Cr (CO)6 in the presence of certain molecules M, can give rise to the following reaction sequence: Suppose that the absorbed light intensity is so weak that! «k4 [Cr (CO) 5M]....
-
Many enzyme-catalyzed reactions are consistent with a modified version of the Michaelis-Menten mechanism in which the second step is also reversible. (a) For this mechanism show that the rate of...
-
Series of Compound Interest Techniques The following are several situations involving compound interest. Required: Using the appropriate table, solve each of the following: ( Click here to access the...
-
If Clark Kelly has recognized gain on an exchange of like-kind property held for investment use, where does Clark report the gain? First on Form 8824, then carried to Schedule D. First on Form 8824,...
-
An investor put 40% of her money in Stock A and 60% in Stock B. Stock A has a beta of 1.2 and Stock B has a beta of 1.6. If the risk-free rate is 5% and the expected return on the market is 12%,...

Study smarter with the SolutionInn App


