Question: What must be added to a double bond to transform it into an alcohol? Would this be an example of oxidation or reduction? (See Figure
What must be added to a double bond to transform it into an alcohol? Would this be an example of oxidation or reduction? (See Figure 18.20)
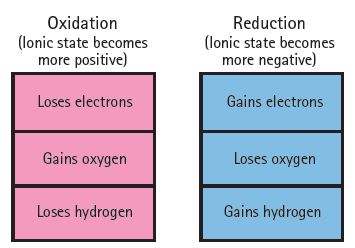
Oxidation (lonic state becomes more positive) Reduction (lonic state becomes more negative) Loses electrons Gains electrons Gains oxygen Loses oxygen Gains hydrogen Loses hydrogen
Step by Step Solution
3.40 Rating (166 Votes )
There are 3 Steps involved in it
Adding a water molecule to ... View full answer

Get step-by-step solutions from verified subject matter experts


