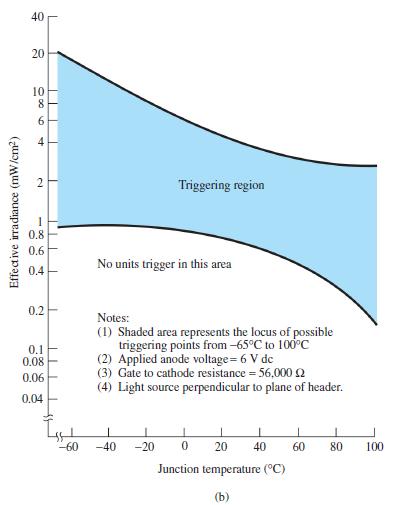
a. Using Fig. 17.24b, determine the minimum irradiance required to fire the device at room temperature (25C).
Question:
a. Using Fig. 17.24b, determine the minimum irradiance required to fire the device at room temperature (25°C).
b. What percentage reduction in irradiance is allowable if the junction temperature is increased from 0°C (32°F) to 100°C (212°F)?
Fig. 17.24b
Transcribed Image Text:
40 20 10 Triggering region 0.8 0.6 No units trigger in this area 0.4 0.2 Notes: (1) Shaded area represents the locus of possible triggering points from -65°C to 100°C (2) Applied anode voltage= 6 V de (3) Gate to cathode resistance = 56,000 (4) Light source perpendicular to plane of header. 0.1 0.08 0.06 0.04 -60 -40 -20 20 40 60 80 100 Junction temperature (°C) (b) TIL | 2. Effective irradiance (mW/cm2)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (10 reviews)
a Using Fig 1724b determine the minimum irradiance required to fire the device at room temper...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Electronic Devices And Circuit Theory
ISBN: 9781292025636
11th Edition
Authors: Robert Boylestad, Louis Nashelsky
Question Posted:
Students also viewed these Engineering questions
-
A room is 25 feet by 32 feet. How much will it cost to cover the floor with carpet costing $12 a square yard (9 square feet), if 4 extra square yards are needed for matching? If a portion of a...
-
If you place water at room temperature in a well-insulated cup and allow some of the water to evaporate, the temperature of the water in the cup will drop lower than room temperature. Come up with an...
-
What temperature is required to obtain 0.50% C at a distance of 0.5 mm beneath the surface of a 0.20% C steel in 2 h, when 1.10% C is present at the surface? Assume that the iron is FCC?
-
Why do the requirements drift once a project is under way?
-
What is the user cost of capital?
-
1. On June 1, 2008, a U.S. firm contracts to sell equipment (with an asking price of 2,000,000 krona) in Sweden. The firm will take delivery and will pay for the equipment on August 1, 2008. 2. Spot...
-
HVAC energy consumption. A control chart to monitor the installation of a new heating, ventilation, and air conditioning (HVAC) system for a clothing store in Panama City, FL, was presented in...
-
Assume that today is June 11. Your firm is scheduled to pay 500,000 on August 15, 65 days in the future. The current spot is $1.75/, and the 65-day forward rate is $1.73/. You can borrow and lend...
-
Patterson Company is considering two competing investments. The first is for a standard piece of production equipment. The second is for computer-aided manufacturing (CAM) equipment. The investment...
-
The following are the financial statements of Post Corporation and its subsidiary, Sage Company, as at December 31, Year 3: Additional Information ¢ Post purchased 70% of the outstanding shares...
-
For the network of Fig. 17.28, if V BR = 6 V, V = 40 V, R = 10 k, C = 0.2 mF, and V GK (firing potential) = 3 V, determine the time period between energizing the network and the turning on of the S...
-
a. In Fig. 17.22, if V Z = 50 V, determine the maximum possible value the capacitor C 1 can charge to (VGK 0.7 V). b. Determine the approximate discharge time (5t) for R 3 = 20 k. c. Determine the...
-
The temperature coefficient of resistance a in Eq. (25.12) equals the temperature coefficient of resistivity a in Eq. (25.6) only if 1he coefficient of Thermal expansion is small. A cylindrical...
-
Name and define the more common constraints in any given project.
-
Graph the function f(x)=-x+4x-20 State where f(x) is increasing and decreasing. State any absolute extrema (if they exist). Determine the Domain and Range.
-
A residential wiring circuit is shown in the figure. In thismodel, the resistor R 3 is used to model a 250 V appliance(such as an electric range), and the resistors R 1 and R 2 are used to model 125...
-
1. The speed limit on some interstate highways is roughly 100 km/h. (a) What is this in meters per second? (b) How many miles per hour is this? 2. A car is traveling at a speed of 33 m/s. (a) What is...
-
Questions 33 and 34 are based on the following information: Bilog Company's budgeted fixed overhead costs are P50,000 and mthe variable factory overhead rate is P4 per direct labor hour. The standard...
-
Barkins Moving Company specializes in hauling heavy goods over long distances. The companys revenues and expenses depend on revenue kilometres, a measure that combines both weights and distance...
-
For each equation, (a) Write it in slope-intercept form (b) Give the slope of the line (c) Give the y-intercept (d) Graph the line. 7x - 3y = 3
-
(a) Write a balanced equation for the reaction and calculate E for the reaction. (b) Predict whether an equimolar mixture of PuO 2 2 + and PuO + 2 will oxidize H 2 O to O 2 at a pH of 2.00 and P O2 =...
-
Calculate the voltage of the following cell, in which KHP is potassium hydrogen phthalate, the monopotassium salt of phthalic acid. By the reasoning in Figure 13-8, in which direction do electrons...
-
The following cell has a voltage of 0.083 V: Hg(l) | Hg(NO3)2(0.001 0 M), KI(0.500 M) || S.H.E. From this voltage, calculate the equilibrium constant for the reaction In 0.5 M KI, virtually all the...
-
Estimate the intrinsic value of the stock company ABC. Dividends were just paid at $8 per share and are expected to grow by 5%. You require 20% on this stock given its volatile characteristics. If...
-
Crane, Inc., a resort management company, is refurbishing one of its hotels at a cost of $6,794,207. Management expects that this will lead to additional cash flows of $1,560,000 for the next six...
-
Match each of the following transactions with the applicable internal control principle that is being violated

Study smarter with the SolutionInn App


