The primary purpose of the reformer is to convert methane and water to carbon monoxide and hydrogen
Question:
The primary purpose of the reformer is to convert methane and water to carbon monoxide and hydrogen (Equation 13.1). The extent of this reaction is limited by chemical equilibrium.
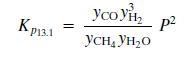
where
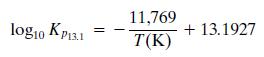
Subscript 13.1 refers to the steam-reforming reaction (Equation 13.1), yi is the mole fraction of species i, P is the system pressure (atm), and T is the temperature (K).
If Equation 13.1 were the only reaction occurring in the reformer, estimate the composition of the product gas that would be leaving the reformer and the conversion of CH , assuming the product stream has achieved chemical equilibrium at 855°\C and 1.6 MPa. What would be the total flow rate of this stream (kmol/h, kg/h)?
It is specified that the molar ratio of steam to methane fed to the reformer is 3.0, whereas the stoichiometric ratio for the reforming reaction (Equation 13.1) is 1 mole of water per mole of methane. Estimate the conversion of methane for steam-to-methane feed ratios of 1:1 and 2:1, and compare these to the conversion in part a. Based on your results, explain in your own words why you think the ratio of 3 moles of steam per mole of methane was chosen for the process.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau





