Vapor pressure data for chlorine are given below. a. Use these data and the ClausiusClapeyron equation (Equation
Question:
Vapor pressure data for chlorine are given below.
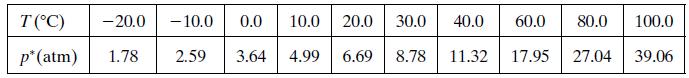
a. Use these data and the Clausius–Clapeyron equation (Equation 6.1-3) to estimate the heat of vaporization of chlorine (kJ/mol) and to obtain an expression for P*Cl2 (T).
b. What is the operating pressure in the chlorine vaporizer (torr)?
c. At what rate (kW) must heat be added to the chlorine in the vaporizer?
Transcribed Image Text:
T(°C) - 20.0 - 10.0 0.0 10.0 20.0 30.0 40.0 60.0 80.0 100.0 p*(atm) 1.78 2.59 3.64 4.99 6.69 8.78 11.32 17.95 27.04 39.06
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 52% (19 reviews)
a The ClausiusClapeyron equation is given by dPdT HvapRT2 where Hvap is the heat of vaporization of ...View the full answer

Answered By

Dulal Roy
As a tutor, I have gained extensive hands-on experience working with students one-on-one and in small group settings. I have developed the ability to effectively assess my students' strengths and weaknesses, and to customize my teaching approach to meet their individual needs.
I am proficient at breaking down complex concepts into simpler, more digestible pieces, and at using a variety of teaching methods (such as visual aids, examples, and interactive exercises) to engage my students and help them understand and retain the material.
I have also gained a lot of experience in providing feedback and guidance to my students, helping them to develop their problem-solving skills and to become more independent learners. Overall, my hands-on experience as a tutor has given me a deep understanding of how to effectively support and encourage students in their learning journey.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau
Question Posted:
Students also viewed these Business questions
-
Estimate the heat of vaporization (kJ/mol) of benzene at a pressure of 100 mm Hg, using each of the following correlations and data: (a) The heat of vaporization at the normal boiling point given in...
-
Estimate the heat of vaporization (kJ/mol) of benzene at 25C, using each of the following correlations and data: (a) The heat of vaporization at the normal boiling point and Watsons correlation. (b)...
-
Estimate the heat of vaporization of ethyl benzene at its normal boiling point using Troutons rule and Chens rule and compare the results with a tabulated value of this quantity. Then estimate Hv at...
-
Calculate the 90% confidence interval for the difference (mu1-mu2) of two population means given the following sampling results. Population 1: sample size = 19, sample mean = 20.52, sample standard...
-
(a) How is a reaction quotient used to determine whether a system is at equilibrium? (b) If Qc > Kc, how must the reaction proceed to reach equilibrium? (c) At the start of a certain reaction, only...
-
Variable manufacturing overhead variance analysis. The French Bread Company bakes baguettes for distribution to upscale grocery stores. The company has two direct-cost categories, direct materials...
-
14. What is the accounting equation for an agency fund?
-
Kirby Railroad Co. is about to issue $300,000 of 10-year bonds paying a 9% interest rate, with interest payable semiannually. The discount rate for such securities is 8%. How much can Kirby expect to...
-
8 If an export sale is denominated in the foreign currency, the exchange risk is assumed by the domestic seller bdomestic buyer c. foreign seller d foreign buyer What is the effect of a strengthening...
-
The following balance sheet for the Los Gatos Corporation was prepared by a recently hired accountant. In reviewing the statement you notice several errors. LOS GATOS CORPORATION Balance Sheet At...
-
The plant is to produce 3.5 10 6 kg/year of "67% Cl." Assuming that the plant operates 300 days per year, 24 hours per day, calculate the required hourly feed rates (kg/h) of the 10 wt% PVC slurry...
-
Vapor pressure data for chlorine are given below. a. Use these data and the ClausiusClapeyron equation (Equation 6.1-3) to estimate the heat of vaporization of chlorine (kJ/mol) and to obtain an...
-
You dont inspect quality into a product; you have to build it in. Discuss the implications of this statement.
-
You have been employed as a systems analyst in the information systems organization of a medium-sized consumer goods manufacturer for three years. You are quite surprised when your manager offers you...
-
For your initial post, address the following: First, introduce yourself to the class by sharing a bit about yourself, such as your preferred name or pronouns, where you are from, what your major is,...
-
Question 8 : Consider the technology of Solar Panels. Which stage of the technology life cycle S curve is this technology in. Justify why ? Question 9 : The standard Product Life Cycle has 5 stages...
-
At Benihana restaurant a man wrenched his neck while ducking a piece of flying shrimp, requiring treatment by several doctors. By that summer, doctors determined surgery was necessary to treat...
-
You have just come into an inheritance of $25,000 from a distant relative, and you want to invest it for the long term. Provide an investment portfolio that includes five different stocks. Report the...
-
Paving brickscalled clinkerswere examined for trace elements in order to determine the origin (e.g., factory) of the clinker. (Advances in Cement Research, Jan. 2004.) The barium content (mg/kg) for...
-
How has the globalization of firms affected the diversity of their employees? Why has increased diversity put an additional burden on accounting systems?
-
A full journal bearing is 25 mm long. The shaft journal has a diameter of 50 mm with a unilateral tolerance of 0.01 mm. The bushing bore has a diameter of 50.05 mm with a unilateral tolerance of 0.01...
-
A 1 - 1 - in sleeve bearing supports a load of 700 lbf and has a journal speed of 3600 rev/min. An SAE 10 oil is used having an average temperature of 160F. Using Fig. 1216, estimate the radial...
-
A full journal bearing has a shaft diameter of 80.00 mm with a unilateral tolerance of 0.01 mm. The l/d ratio is unity. The bushing has a bore diameter of 80.08 mm with a unilateral tolerance of 0.03...
-
On January 1, 2018, Brooks Corporation exchanged $1,259,000 fair-value consideration for all of the outstanding voting stock of Chandler, Inc. At the acquisition date, Chandler had a book value equal...
-
1. Determine the value of the right to use asset and lease liability at commencement of the lease.
-
Problem 22-1 The management of Sunland Instrument Company had concluded, with the concurrence of its independent auditors, that results of operations would be more fairly presented if Sunland changed...

Study smarter with the SolutionInn App


