Consider a transition of X Y. Assume that the only difference between X and Y is
Question:
Consider a transition of X → Y. Assume that the only difference between X and Y is the presence of three hydrogen bonds in Y that are absent in X. What is the ratio of X to Y when the reaction is in equilibrium? Approximate your answer by using Table 3−1 (p. 96), with 4.2 kJ/mole as the energy of each hydrogen bond. If Y instead has six hydrogen bonds that distinguish it from X, how would that change the ratio?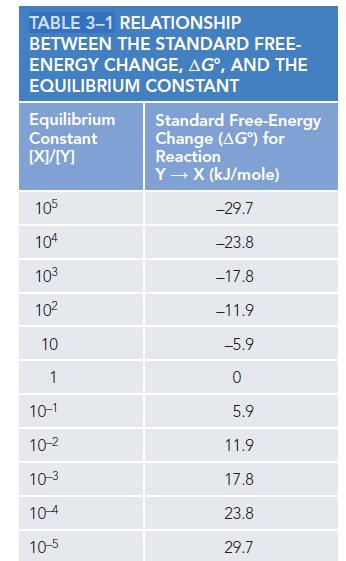
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Essential Cell Biology
ISBN: 9780393680362
5th Edition
Authors: Bruce Alberts, Karen Hopkin, Alexander Johnson, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Question Posted:





