In a simple reaction A A*, a molecule is interconvertible between two forms that differ in
Question:
In a simple reaction A ↔ A*, a molecule is interconvertible between two forms that differ in standard free energy G° by 18 kJ/mole, with A* having the higher G°.
A. Use Table 3–1 to find how many more molecules will be in state A* compared with state A at equilibrium.
B. If an enzyme lowered the activation energy of the reaction by 11.7 kJ/mole, how would the ratio of A to A* change?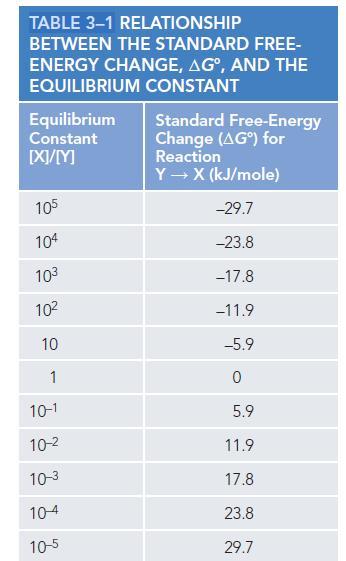
Transcribed Image Text:
TABLE 3-1 RELATIONSHIP BETWEEN THE STANDARD FREE- ENERGY CHANGE, AG°, AND THE EQUILIBRIUM CONSTANT Equilibrium Constant [X]/[M] 105 104 103 10² 10 1 10-1 10-² 10-3 10-4 10-5 Standard Free-Energy Change (AG) for Reaction Y→ X (kJ/mole) -29.7 -23.8 -17.8 -11.9 -5.9 0 5.9 11.9 17.8 23.8 29.7
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
A To find how many more molecules will be in state A compared to state A at equilibrium we need to u...View the full answer

Answered By

Surendar Kumaradevan
I have worked with both teachers and students to offer specialized help with everything from grammar and vocabulary to challenging problem-solving in a range of academic disciplines. For each student's specific needs, I can offer explanations, examples, and practice tasks that will help them better understand complex ideas and develop their skills.
I employ a range of techniques and resources in my engaged, interesting tutoring sessions to keep students motivated and on task. I have the tools necessary to offer students the support and direction they require in order to achieve, whether they need assistance with their homework, test preparation, or simply want to hone their skills in a particular subject area.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Essential Cell Biology
ISBN: 9780393680362
5th Edition
Authors: Bruce Alberts, Karen Hopkin, Alexander Johnson, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Read the case study "Southwest Airlines," found in Part 2 of your textbook. Review the "Guide to Case Analysis" found on pp. CA1 - CA11 of your textbook. (This guide follows the last case in the...
-
Identify a true statement about the rational and emotional aspects of leadership. Multiple choice question. Leadership is not about the rational or emotional sides of human experience Leadership...
-
Data for Virtual Gaming Systems are provided in P124A. Earnings per share for the year ended December 31, 2015, are $1.40. The closing stock price on December 31, 2015, is $28.30. In P124A, The...
-
Suppose that a country experiences a reduction in productivitythat is, an adverse shock to the production function. a. What happens to the labour demand curve? b. How would this change in...
-
Describe a forward exchange contract. LO6
-
Van makes an investment in a partnership in 2014. Vans capital contributions to the partnership consisted of $30,000 cash and a building with an adjusted basis of $70,000, subject to a nonrecourse...
-
Question 12 2 pts A company has 2 departments. Job 82 goes through Department A first at a cost of $10,000 for direct materials, $12,000 for direct labor, and $15,000 for allocated overhead. Then the...
-
You are employed by McDowell and Partners, Chartered Accountants (M&P). A new client, Community Finance Corporation (CFC), approached M&P for assistance. Enviro Ltd. (Enviro) has asked CFC for a loan...
-
Which of the following amino acids would you expect to find more often near the center of a folded globular protein? Which ones would you expect to find more often exposed to the outside? Explain...
-
Neurofilament proteins assemble into long, intermediate filaments, found in abundance running along the length of nerve cell axons. The C-terminal region of these proteins is an unstructured...
-
Over the course of 2015, the first year of operations, Medical Supplies, Inc. had the following income transactions: Sales Revenue of $ 4,340,000; Cost of Goods Sold of $ 1,936,000; Wage Expense of $...
-
Design an arithmetic circuit with two selection variables S 1 and S 0 and two n- bit data inputs A and B. The circuit generates the following eight arithmetic operations in conjunction with carry C...
-
Larrys Sporting Goods is a locally owned store that specializes in printing team jerseys. The majority of its business comes from orders for various local teams and organizations. While Larrys prints...
-
Pecos Pecan Pads makes pressed pecan wood covers to prevent weed growth. During July 2009, the company produced and sold 44,000 rolls and recorded the following cost data: Requirements 1. Compute the...
-
The Human Resources departments costs are allocated to the other departments based on the number of direct labor hours. The departments expected fixed costs are 400,000 and its variable costs are...
-
A lawyer allocates overhead costs based on her hours working with different clients. The lawyer expects to have \($200,000\) in overhead during the year and expects to work on clients cases 2,000...
-
Define the following terms: IRR, use of Cost of Capital, Cost of Equity, Cost of Debt, Cost of Preferred Stock, Weighted Average Cost of Capital.
-
What are the principal alloying elements in SAE 4340 steel?
-
In each case, identify the more stable anion. Explain why it is more stable. (a) (b) (c) vs. N. vs. -zo
-
Atropine, extracted from the plant Atropa belladonna, has been used in the treatment of bradycardia (low heart rate) and cardiac arrest. Draw the enantiomer of atropine: CH 0= -
-
Recall that a C=C bond is comprised of a s bond and a p bond. These two bonds together have a combined BDE (bond dissociation energy) of 632 kJ/mol. Use this information to predict whether the...
-
The major justification for adding Step 0 to the U.S. GAAP impairment test for goodwill and indefinite lived intangibles is that it: A. Saves money spent estimating fair values B. Results in more...
-
Regarding research and experimental expenditures, which of the following are not qualified expenditures? 3 a. costs of ordinary testing of materials b. costs to develop a plant process c. costs of...
-
Port Ormond Carpet Company manufactures carpets. Fiber is placed in process in the Spinning Department, where it is spun into yarn. The output of the Spinning Department is transferred to the Tufting...

Study smarter with the SolutionInn App


