Iron-56, with nuclear mass 55.9206 u, is among the most tightly bound nuclei. Find the binding energy
Question:
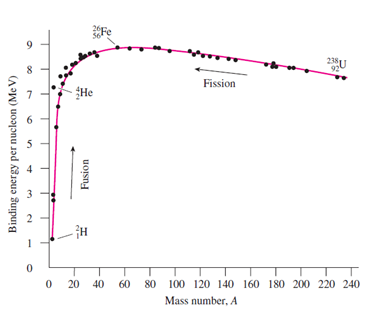
Iron-56, with nuclear mass 55.9206 u, is among the most tightly bound nuclei. Find the binding energy per nucleon, and check your answer against Fig. 38.9.
Transcribed Image Text:
20Fe 8. 92 Fission He 5 20 40 60 80 100 120 140 160 180 200 220 240 Mass number, A Fusion 6) 3. 2. Binding energy per nucleon (Me V)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
Given Nuclear mass of 56Iron 559206 u The iron has 26 protons There...View the full answer

Answered By

Chandan Bagdia
After finishing my high school education I realized that I like physics. Probably because it connects you with the understanding of the world that we see around us. Hence, I decided to join the India Institute of Technology Delhi (IIT Delhi) for my bachelors in Engineering Physics. The course made me curious to know dipper in physics. Hence I decided to join as a research scholar in Tata Institute of Fundamental Research, Mumbai (TIFR). Currently, I am a final year PhD scholar in the Department of Nuclear and Atomic Physics (DNAP).
I have experience of about 2 years to teach a student Physics. Apart from that, I have been a teaching assistant for the Atomic collisions course in TIFR. Apart from these I really like to teach and solve problems. Solving problems gives me immense joy.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The binding energy per nucleon in the iron nucleus 2566 Fe is 8.8 MeV. Find its atomic mass.
-
Verify the binding energy per nucleon given in Table for 239pu. The mass of the atom is 239.052 16u. Some Properties of Selected Nuclides Mass Binding Energy (MeV/nucleon) Spin Nuclide Stability (u)...
-
The binding energy per nucleon for magnesium-27 is 1.326 X 10-12 J/nucleon. Calculate the atomic mass of magnesium-27.
-
(a) An n n matrix K is the encryption matrix for the Hill cipher. Give the encryption formula. (b) The plaintext is (2, 5, 1,0). Encrypt it (show your work and the ciphertext) using the Hill cipher...
-
The boiling poins of the l,2-dichloroethylene stereoisomers are47.4oCand 60.3C. Give the structure of the stereoisomer with the higher boiling point. Explain.
-
Explain the following properties of silicon(IV) oxide by referring to its structure and bonding. a. It has a high melting point. b. It does not conduct electricity. c. It is a crystalline solid. d....
-
5. What are technical constraints? Give some examples.
-
On January 1, 2013, Hatch Co. borrowed $100,000 cash from First Bank by issuing a four-year,6percent note. The principal and interest are to be paid by making annual payments in the amount of...
-
Imperial Jewelers manufactures and sells a gold bracelet for $410.00. The company's accounting system says that the unit product cost for this bracelet is $258.00 as shown below: Direct materials...
-
The Teachers' Retirement System of a midwestern state is selling a bond investment from its portfolio to generate cash to make payments to retirees for the coming year It plans to sell $100 million...
-
An NMR spectrometer is described as a 300-MHz instrument, meaning 3.00x10 8 Hz is the frequency supplied to its transmitter coil to flip the spin states of bare protons. Whats the strength of its...
-
Find the atomic mass of iridium-193, whose binding energy is 7.94 MeV/nucleon.
-
Compute the current ratio and acid-test ratio for each of the following separate cases. Which com pany case is in the best position to meet short-term obligations? Explain. lpo5 Case X Case Y Case Z...
-
Define an interface TwoStrings Oper declaring a function apply which takes two strings and returns a string. Then, define four classes implementing this interface, where the operation on strings...
-
Noeleen AutoMall, Ltd. recently completed an initial public offering(IPO) for$23,000,000 by listing its common shares on the New York Stock Exchange. Prior to itsIPO, Noeleen was a privately held...
-
Process Costing- increased units, FIFO method Answer in good form 25 26 Illustrative Problem-Cost of Production Report using Treatment by Neglect Dept 1-100% of materials are added at the beginning....
-
Write a C++ program that prompts the user to enter a letter and encrypt it using the following method: if the letter is an upper-case letter the program replaces the letter by the 7th letter in the...
-
Turn this information into an excel sheets with the excel formulas being shown P12.4 (LO 1) (Payroll Tax Entries) The following is a payroll sheet for Otis Imports for the month of September 2025....
-
Figure 18.4 shows the call theta as a function of time to maturity. Produce a similar graph for puts. Call Theta, Time to Maturity, and Moneyness 01 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0 Time to optlon...
-
A superior criticized a sales manager for selling high-revenue, low-profit items instead of lower-revenue but higher-profit items. The sales manager responded, My income is based on commissions that...
-
The composite shaft, consisting of aluminum, copper, and steel sections, is subjected to the loading shown. Determine the displacement of end A with respect to end D and the normal stress in each...
-
The copper shaft is subjected to the axial loads shown. Determine the displacement of end A with respect to end D if the diameters of each segment are d AB = 0.75 in., d BC = 1 in., and d CD = 0.5...
-
The A992 steel rod is subjected to the loading shown. If the cross-sectional area of the rod is 80 mm 2 , determine the displacement of B and A. Neglect the size of the couplings at B and C. 0,75 m...
-
According to Barth, Caprio, and Levine, regulators ought to think of ways of helping financial markets, particularly bank debt and equity holders, to monitor banks. 1) True 2) False VE
-
Suppose you see that a stock has a very high Price-to-earnings (P/E) ratio. Does it imply that this stock is overvalued? Why or why not? Explain
-
Statements on Auditing Standards (SAS) provide more detailed guidance of: a. PCAOB assertions b. statutory law c. GAAS d. GAAP

Study smarter with the SolutionInn App


