Lithium fluoride, LiF, has the same crystal structure as NaCl and therefore has essentially the same Madelung
Question:
Lithium fluoride, LiF, has the same crystal structure as NaCl and therefore has essentially the same Madelung constant a. Its ionic cohesive energy is -10.5 eV and the value of n in Equation 37.4 is 6.25 Find equilibrium ionic separation LiF.?
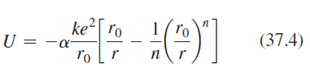
Transcribed Image Text:
ke ro U = (37.4) ľo [r
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
The equilibrium ionic separation in LiF can be determined using the Madelun...View the full answer

Answered By

Shahid Akbar
In my point of view, Education can't be finished when it's shared even it will increase in quantity. I like to share my knowledge to everyone who has the interest to improve his skills.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Determine the constant n in Equation 37.4 for potassium chloride (KCl), which has the same crystal structure as NaCl and for which r 0 = 0.315 nm and U 0 = -7.21 eV. ke ro 1 (37.4) U = ro [r
-
The theoretical density of germanium is 5.324 g/cm3 at 300 K. Germanium has the same crystal structure as diamond. What is the lattice constant of germanium at 300 K?
-
Find the value of n?in Equation 38-32 that gives the measured dissociation energy of 741 kJ/mol for LiCl, which has the same structure as NaCl and for which r 0 = 0.257 nm. ke? (1-) U(r.) = -a- 38-32
-
Describe the risk assessment approach used for Enterprise Risk Management(ERM) at Worker's Compensation Fund(WCF). How does this approach compare to ISO 31000? Does it consider risks with upside...
-
Design a preparation of each of the following compounds from an alcohol using sulfonate ester methodology- CH,CH,CH2 SCH
-
Prepare a bank reconciliation statement from the following information, clearly identifying the balance as per the bank statement. Credit balance as per cash book 1912.86 Unpresented cheques...
-
l Describe the agency problem and explain how this problem may be dealt with.
-
The financial statements of The Hershey Company appear in Appendix B, following the financial statements for Tootsie Roll in Appendix A. Instructions (a) Based on the information contained in these...
-
On January 1 , 2 0 2 3 , Novak Corporation, a public company following IFRS, acquired 1 5 , 9 0 0 of the 5 3 , 0 0 0 outstanding common shares of Noah Corp. for $ 2 2 per share. Noah's statement of...
-
Refer to Exercise 29 of Section 4.2. For what values of h will a sensitivity analysis on the effect of a change of h pounds of peat be valid? In exercise A lawn and garden store creates three...
-
Express the 7.84-eV ionic cohesive energy of NaCl in kilocalories per mole of ions.
-
Find the equilibrium ionic separation in LiF. 25. Find the wavelength of light emitted by a gallium phosphide (GaP) light-emitting diode. (See Table 37.1.) Table 37.1 Band-Gap Energies for Selected...
-
Suppose your company purchased land and a warehouse for $5 million. The price was steep, but you were told that a new interstate highway was going to be built nearby. Two months later, the highway...
-
A steady flow of 20 m3/s of moist air at TDB = 35iC, TWB = 25iC, 100 kPa (state 1) is dehumidified by first cooling it and condensing out moisture (state 2), then reheating it to 20iC and 50% R.H....
-
Question 37 Plantito Inc., produces potted plants. For next year, Pietro predicts that 45,000 units will be produced, with the following total costs: Direct materials Direct labor ? 80,000 Variable...
-
When you are to design a data transmission system, you have two key considerations to work with: data rate and distance, with emphasis placed on achieving the highest data rates over the longest...
-
How much work does a supermarket checkout attendant do on a can of soup he pushes 0.600 m horizontally with a force of 5.00 N? Express your answer in joules and kilocalories. 3 . (a) Calculate the...
-
Suppose in its income statement for the year ended June 30, 2022, The Clorox Company reported the following condensed data (dollars in millions). Salaries and wages expenses$460 Research and...
-
Rewrite TransposeMeToo of exercise 8 so it could deal with all ranges. Rewrite exercise 8 Array functions are functions that return more than one value. For example, the Transpose worksheet function...
-
From the choice of simple moving average, exponential smoothing, and linear regression analysis, which forecasting technique would you consider the most accurate? Why? please write it in word...
-
The pump operates using the motor that has a power of 85 W. If the impeller at B is turning at 150 rev/min, determine the maximum shear stress in the 20-mm-diameter transmission shaft at A. 150...
-
The solid steel shaft AC has a diameter of 25 mm and is supported by smooth bearings at D and E. It is coupled to a motor at C, which delivers 3 kW of power to the shaft while it is turning at 50...
-
The motor delivers 50 hp while turning at a constant rate of 1350 rpm at A. Using the belt and pulley system this loading is delivered to the steel blower shaft BC. Determine to the nearest 1/8 in....
-
Robert and Sherrell Bergeron gave a first mortgage on their property to First Colonial Bank and a second mortgage to Ford Motor Credit Company. When the Bergerons were unable to pay the mortgage, the...
-
DOTC had the following accounts balance as of 2021: Current Assets P 14,000,000 Investment and Fixed Assets P 126,000 Other Assets P 7,000,000 Liabilities P 25,200,000 Contingent Liabilities P...
-
Assume an investor buys a newly issued 8 percent, semiannual 10 year bond at par. He sells it two years later when market interest rates have decreased to 6 percent. How much is the investors capital...

Study smarter with the SolutionInn App


