Thorium-232 is an a emitter with 14-billion-year half-life. Radium-228 is a ? - emitter with 5.75-year half-life.
Question:
Thorium-232 is an a emitter with 14-billion-year half-life. Radium-228 is a ?- emitter with 5.75-year half-life. Actinium-228 is a ?- emitter with 6.13-hour half-life.(a) What?s the third daughter in the thorium-232 decay series?(b) Make a chart similar to Fig. 38.7 showing the first three decays in the thorium series.
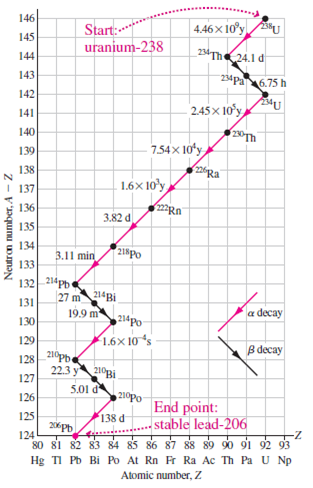
Transcribed Image Text:
146 Start:* 4.46 x 10°y 145 uranjum-238 144 234Th24.1 d 143 234p6.75 h 142 141 2.45x 10'y 140 139 7.54x 10ʻy 138 226p Ra I 137 1.6x 10'y 136 3.82 d 135 134 218Po 3.11 min 133 132 214Pb 27 m 214Bi 131 19.9 m a decay 130 214po 129 1.6x 10 s _210p B decay 128 22.3 y 210Bi 127 5.01 d 126 210po End point: stable lead-206 125 138 d 124 80 81 82 83 84 85 86 87 88 89 90 91 92 93 Hg TI Pb Bi Po At Rn F Ra Ac Th Pa U Np -- Atomic number, Z Neutron number, A - Z
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 68% (16 reviews)
a The third daughter in the thorium232 decay series is Radium228 b The chart showing the first three ...View the full answer

Answered By

Ravi Ravi
I have been an online tutor for almost two years now and I love it every time my students praise me for my work and tell me that I have helped them in their homework and projects. It gives me a sense of pride every time a student recognizes the good assistance that I have provided for them.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Thorium-232 (23290Th) decays via a decay. Write out the reaction and identify the daughter nuclide.
-
In the thorium decay series, thorium-232 loses a total of 6 particles and 4 particles in a 10-stage process. What is the final isotope produced?
-
Actinium-227 decays by alpha decay or beta decay and is part of a long decay sequence, shown in Fig. 29.8. Write all the possible nuclear decays for the decay series from 227Ac to 215Po. Identify the...
-
The following is information for a perfectly price discriminating monopolist. Demand: P = 65 0.02Q Marginal revenue = P = 65 0.04Q Marginal cost = ATC = 4 Calculate the producer surplus for the...
-
It has been argued that the energy difference between crs- and trans-1,3-di-tert-butylcyclohexane is a good approximation for the energy difference between the chair an4 twist-boat forms of...
-
The table shows the type of bonding in a number of elements and compounds. a. Draw a labelled diagram to show metallic bonding. b. Explain why magnesium chloride has a high melting point but bromine...
-
Prepare a resource requirements plan
-
As a preliminary to requesting budget estimates of sales, costs, and expenses for the fiscal year beginning January 1, 20Y9, the following tentative trial balance as of December 31, 20Y8, is prepared...
-
The Polishing Department of Ivanhoe Company has the following production and manufacturing cost data for September. All materials are added at the beginning of the process, and conversion costs are...
-
Presented below is the unadjusted trial balance of Webster Demolition Company as of June 30, 2023, the end of its fiscal year. The owner invested $17,500 cash in the company during the year. Required...
-
Nitrogen-13 is a 9.97-min-half-life isotope used to tag ammonia for PET scans, including quantification of myocardial infarction. Consider an intravenous injection incorporating 20.0 mCi of N-13....
-
How much cobalt-60 (t 1/2 = 5.24 years) must be used to make a laboratory source whose activity will exceed 1 GBq for 2 years?
-
An experiment is conducted to compare the starting salaries of male and female college graduates who find jobs. Pairs are formed by choosing a male and a female with the same major and similar grade...
-
Question 3 58.5 Average global temperature 1880-2013 58.0 $ 57.5 57.0 56.5 1880 1900 1920 1940 1960 1980 2000 2020 Year The graph above indicates that global temperatures have Ovaried randomly over...
-
In 2022, Andrew, who is single, has a comfortable salary from his job as well as income from his investment portfolio. However, he is habitually late in filing his federal income tax return. He did...
-
1. What is the cost of direct materials used? 2. What is the cost of indirect materials used? 3. What is the cost of direct labour? 4. What is the cost of indirect labour? 5. What is the cost of...
-
Finding Critical Values. In Exercises 5-8, find the critical value za/2 that corresponds to the given confidence level. 5. 90% 6. 99%
-
You are an attorney at the law firm that represents Danfield's Auto Express. Your supervisor, Attorney Donna Defense, wants you to draft an internal memorandum of law to her assessing whether or not...
-
Here?s an advanced version of exercise 10. Consider an alternative parameterization of the binomial: Construct binomial European call and put option pricing functions in VBA for this parameterization...
-
Which of the companies has the lowest accounts receivable turnover in the year 20X2? a. Company A. b. Company B. c. Company C. d. CompanyD. 20X1 20X2 Credit Sales Average Receivables Balance $1.0...
-
Determine the elongation of the bar in Prob. 4108 when both the load P and the supports are removed. B -2 ft -3 ft
-
The bar having a diameter of 2 in. is fixed connected at its ends and supports the axial load P. If the material is elastic perfectly plastic as shown by the stressstrain diagram, determine the...
-
The rigid beam is supported by the three posts A, B, and C. Posts A and C have a diameter of 60 mm and are made of a material for which E = 70 GPa and Ï Y = 20 MPa. Post B is made of a material...
-
which of the following actions would improve a firms liquidity? A, buying machinery with long term debt. B, purchasing inventories for cash, C, purchasing inventory on trade credit, D, purchasing...
-
32 Financial managers: are described by none of the above are not typically involved in long-term strategic planning are simply a type of bookkeeper focus on profit maximization play such an...
-
During 2019 and 2018, the company acquired five businesses for an aggregate consideration of $440.4 million (net of cash acquired), one of which was Pro. Exhibit 6 provides information about Pro as...

Study smarter with the SolutionInn App


