The table shows the type of bonding in a number of elements and compounds. a. Draw a
Question:
The table shows the type of bonding in a number of elements and compounds.
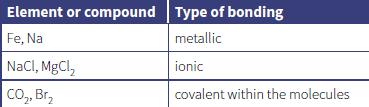
a. Draw a labelled diagram to show metallic bonding.
b. Explain why magnesium chloride has a high melting point but bromine has a low melting point.
c. Explain why solid sodium conducts electricity but solid sodium chloride does not conduct electricity.
d. i. Draw a dot-and-cross diagram for carbon dioxide.
ii. Describe the shape of the carbon dioxide molecule.
iii. Explain why a carbon dioxide molecule has this shape.
e. Bromine is a liquid at room temperature. Weak van der Waals’ forces hold the bromine molecules together. Describe how van der Waals’ forces arise.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:





