Question: A cell mono layer used in a tissue engineering scaffold adheres onto the top surface of a silicone rubber (polymer) sheet of 0.10 cm thickness,
A cell mono layer used in a tissue engineering scaffold adheres onto the top surface of a silicone rubber (polymer) sheet of 0.10 cm thickness, as shown in the figure below. The rectangular sheet is 5.0 cm by 10.0 cm. The underside of the silicone polymer layer is in contact with pure O2gas. The O2gas dissolves into the polymer and diffuses through the polymer to the adhered cells to deliver oxygen to them. The solubility of dissolved O2in the silicone polymer is defined by a linear relationship pA= C'A/S, where pAis the partial pressure of O2gas (atm), S is the solubility constant of O2dissolved in the silicone polymer (S = 3.15 × 10-3mmole O2/cm3· atm at 25ºC), and C'Ais the concentration of O2dissolved in the silicone rubber (mmole O2/cm3). The process is isothermal at 25oC. The molecular diffusion coefficient of O2in silicone rubber is 1 × 10-7cm2/s at 25oC. It is assumed that (1) the cells are oxygen-starved, and so any O2that reaches the cell layer is immediately consumed and that (2) the cells consume O2by a zero-order process that is not dependent on dissolved O2concentration. It is determined that the sustainable O2consumption rate of the cell mono layer is fixed at 1.42 × 10€“5O2mmole O2/min (0.0142 μmole O2/min). What the required O2partial pressure (pA) required to for the diffusion process to enable this O2consumption rate?
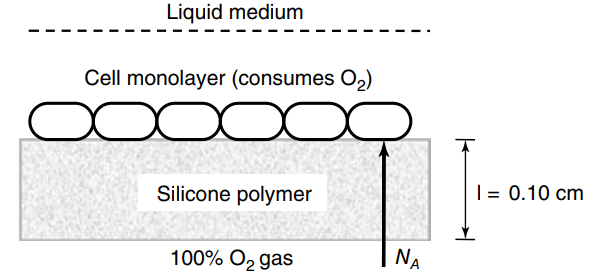
Liquid medium Cell monolayer (consumes O2) | = 0.10 cm Silicone polymer NA 100% O2 gas
Step by Step Solution
3.41 Rating (170 Votes )
There are 3 Steps involved in it
A O 2 B silicone polymer layer Find p A given W A Assume ... View full answer

Get step-by-step solutions from verified subject matter experts


