Question: A flat surface containing many parallel pores is clogged with coke from a manufacturing process, as shown in the figure below. Pure oxygen gas (O
A flat surface containing many parallel pores is clogged with €œcoke€ from a manufacturing process, as shown in the figure below. Pure oxygen gas (O2) at high temperature is used to oxidize the coke, which is mainly solid carbon, to carbon dioxide (CO2) gas. This process will remove the solid carbon clogging the pores, and hence clean the surface. A large excess of O2is in the bulk gas over the surface, and so it may be assumed that bulk gas composition is always 100% O2. It may also be assumed that the oxidation reaction is very rapid relative to the rate of diffusion, so that the production of CO2is limited by mass transfer, and the O2concentration at the carbon surface is essentially zero. The pores are cylindrical, with diameter of 1.0 mm and depth of 5 mm. The oxidation process is carried out at 2.0 atm total system pressure and 600°C. The density of solid carbon is 2.25 g/cm3. Let A = O2, and B = CO2.
a. At some time after the oxidation process, the cleaned depth of the pore is 3.0 mm (0.3 cm) from the mouth of the pore. What is the total emissions rate of CO2 gas (WB) at this point in the process?
b. How long will the oxidation process take to reach this cleaned depth of 0.3 cm from the mouth of the pore?
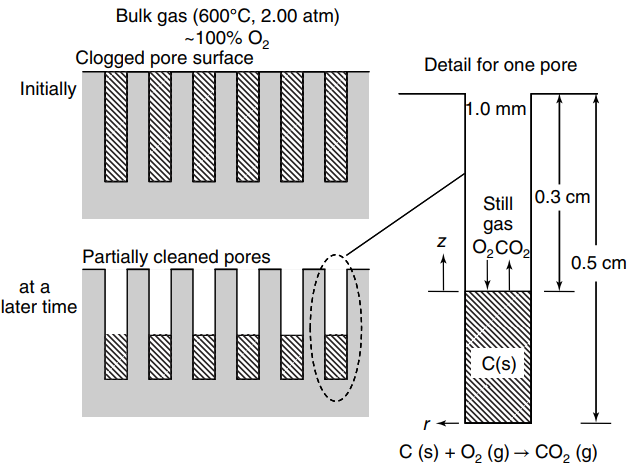
Bulk gas (600C, 2.00 atm) -100% O2 Clogged pore surface Detail for one pore Initially 1.0 mm Still 0.3 cm gas 0,CO2 Partially cleaned pores 0.5 cm at a later time C(s) C (s) + O2 (g) CO, (g)
Step by Step Solution
3.43 Rating (162 Votes )
There are 3 Steps involved in it
Assume 1 PSS system 2 No homogeneous reaction 3 1D flux along z EMCD a Determine W B ... View full answer

Get step-by-step solutions from verified subject matter experts


