Question: The Arnold Diffusion Cell shown in Figure 26.5 is a simple device used to measure gas-phase diffusion coefficients for volatile substrates in air. In the
The Arnold Diffusion Cell shown in Figure 26.5 is a simple device used to measure gas-phase diffusion coefficients for volatile substrates in air. In the present experiment, liquid acetone is loaded to the bottom of a glass tube of 3.0 mm inner diameter. The tube and the liquid acetone in the tube are maintained at a constant temperature of 20.9oC. The tube is open to the atmosphere, and air blows over the open end of the tube, but the gas space inside the cylindrical tube is still. The total length of the tube is 15.0 cm. As the acetone evaporates, the liquid level decreases, which increases Z, the diffusion path length in the gas from the liquid surface to the mouth of the tube. Measurements for the diffusion path length (Z) as a function of time (t) are presented in the table below:
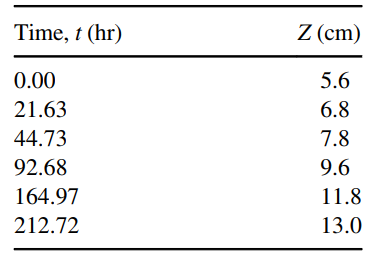
a. Manipulate the data shown in the table above so that it can be plotted as a straight line. Statistically estimate the slope of this line by least-squares linear regression, and then use the slope to estimate the diffusion coefficient of acetone in air.
b. Compare the result in part (a) to an estimate of diffusion coefficient of acetone in air by a suitable correlation given in Chapter 24.
Potentially useful data: The molecular weight of acetone (MA) is 58 g/gmole; the density of liquid acetone (ÏA,liq) is 0.79 g/cm3; the vapor pressure of acetone (PA*) at 20.9°C is 193 mm Hg.
Figure 26.5
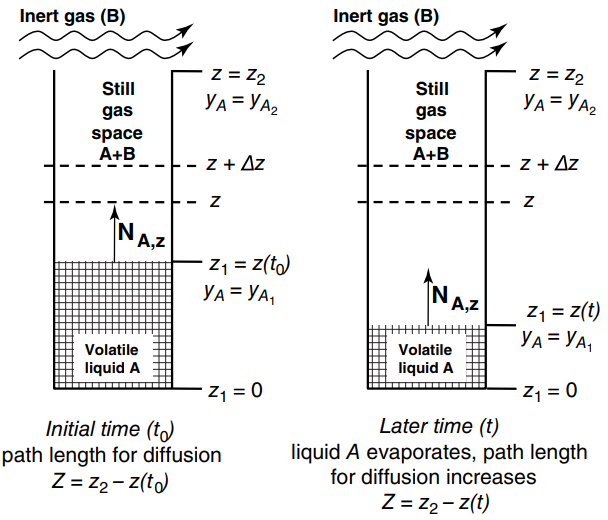
Z (cm) Time, t (hr) 5.6 0.00 21.63 6.8 44.73 7.8 92.68 9.6 164.97 11.8 212.72 13.0 Inert gas (B) Inert gas (B) Z = Z2 - 2 Z = Z2 %3D2 Still Still gas gas space A+B space A+B Z + Az - Z + Az NAZ Z1 = z(to) A 3D , A,Z Z1 = z(t) 3 , Volatile Volatile liquid A liquid A Z1 = 0 Z1 = 0 Initial time (t) path length for diffusion Z = z2- z(to) Later time (t) liquid A evaporates, path length for diffusion increases Z = z2- z(t) N.
Step by Step Solution
3.38 Rating (170 Votes )
There are 3 Steps involved in it
A acetone B air d 03 cm T 2939 K a Determine D AB from Arnold D... View full answer

Get step-by-step solutions from verified subject matter experts


