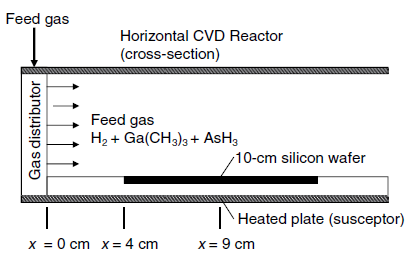
A horizontal chemical vapor deposition (CVD) reactor for growth of gallium arsenide (GaAs) thin films is shown
Question:
2 AsH3 (g) †’ 2 As (s) + 3 H2 (g)
2 Ga(CH3)3 + 3H2 (g) †’ 2 Ga(s) + 6 CH4 (g)
If the process is considerably diluted in H2 gas, then the mass transfer of each species in the H2 carrier gas can be treated separately. The surface reaction is very rapid, and so the mass transfer of the gaseous reactants to the surface of the wafer limits the rate of GaAs thin film formation. In the present process, the edge of a 10.0 cm silicon wafer is positioned 4.0 cm downstream of the leading edge of the susceptor plate. The wafer is inset within this plate so that a contiguous flat surface is maintained.
The process temperature is 800 K, and the total system pressure 101.3 kPa (1.0 atm). Consider a limiting case where the flow rate of the H2-rich feed gas to the reactor results in a bulk linear velocity of 100 cm/s, where trimethylgallium is present in dilute concentration. Determine the local mass-transfer coefficient (kc) for trimethylgallium in H2 gas at the center of the wafer using boundary-layer theory. The binary gas-phase diffusion coefficient of trimethylgallium in H2 is 1.55 cm2/s at 800 K and 1.0 atm.
Step by Step Answer:

Fundamentals Of Momentum Heat And Mass Transfer
ISBN: 9781118947463
6th Edition
Authors: James Welty, Gregory L. Rorrer, David G. Foster





