Consider an inter phase mass-transfer process for the chlorine dioxide (C1O 2 )-air-water system at 20°C, where
Question:
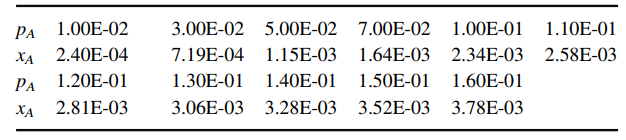
a. Plot out the equilibrium line in pA €“ cAL coordinates, and the operating point (pA, cAL). Is the process gas absorption or liquid stripping?
b. What is the equilibrium relationship as m equal to?
c. What is kL for the liquid film?
d. If the C1O2 mole fraction in the bulk gas phase is maintained at 0.040 under 1.5 atm total system pressure, what is the maximum possible dissolved C1O2 concentration (gmole A/m3) in the liquid phase that could possibly exit the process€”i.e., c*AL?
e. What are the compositions at the gas€“liquid interface, pA,i and cAL,i?
f. What is Ky the overall mass-transfer coefficient based upon the overall gas phase mole fraction driving force? There are several valid approaches for calculating Ky, based on the information provided. Show at least two approaches that lead to the same result.
g. What is the mass-transfer flux NA for C1O2 in units of gmo1e/m2 · s?
DistributionThe word "distribution" has several meanings in the financial world, most of them pertaining to the payment of assets from a fund, account, or individual security to an investor or beneficiary. Retirement account distributions are among the most...
Step by Step Answer:

Fundamentals Of Momentum Heat And Mass Transfer
ISBN: 9781118947463
6th Edition
Authors: James Welty, Gregory L. Rorrer, David G. Foster





