Question: Recall from example 3, Chapter 25, that the differential model for the radial concentration profile of dissolved oxygen within one cylindrical engineered tissue bundle (Figure
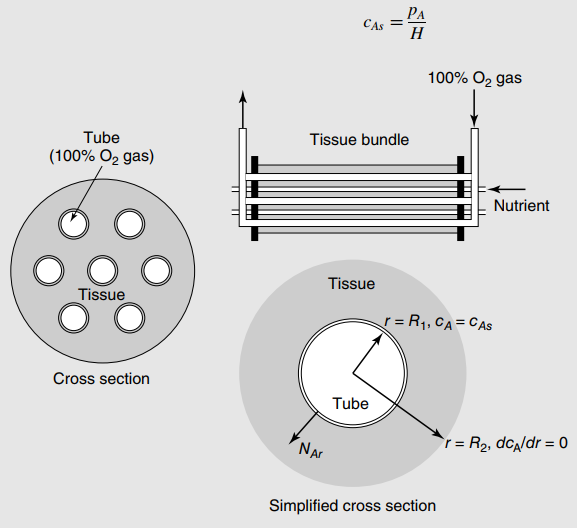
Recall from example 3, Chapter 25, that the differential model for the radial concentration profile of dissolved oxygen within one cylindrical engineered tissue bundle (Figure 25.8) is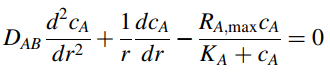
with boundary conditions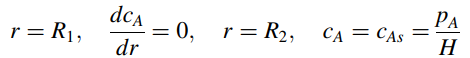
Often, KA is very small relative to cA so that the homogeneous reaction term approaches a zero-order process that is not dependent on concentration:
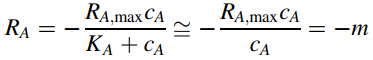
so that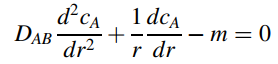
where m is the metabolic respiration rate of the tissue. In the present process, in = 0.25 mole/m3 · h at 25°C, and R1 and R2 are equal to 0.25 cm and 0.75 cm, respectively. Pure O2 gas at 1.0 atm flows through the tube of length 15 cm. The mass- transfer resistance due to the thin-walled tube is neglected, and the Henry€™s law constant for dissolution of O2 in the tissue is 0.78 atm · m3/mole at 25°C. The diffusion coefficient of oxygen in water is 2.1 · 10-5 cm2/s at 25°C, which approximates the diffusivity of oxygen dissolved in the tissue.
a. Develop a model, in final integrated form, to predict the concentration profile cA(r), and then plot out the concentration profile. Note that for diffusion with zero-order homo geneous chemical reaction, there will be a critical radius, Rc, where the dissolved oxygen concentration goes to zero. Therefore, if Rc 2, then cA (r) = 0 from r = Rc to r = R2.
b. Using this model and the process input parameter detailed above, determine Rc. Then, plot of the concentration profile from cA(r) from r = R1 to r = Rc.
c. Develop a model, in final algebraic form, to predict WA, total oxygen transfer rate through one tube. From the process input parameters given in the problem statement, calculate WA.
Figure 25.8
d'cA DAB 1 dca RA,maxCA KA + CA 0 = r dr dr? PA r = R1, dca = 0, dr r = R2, CA = CAS
Step by Step Solution
3.47 Rating (157 Votes )
There are 3 Steps involved in it
A O 2 B tissue approximates liquid water a Model to predict c A r From Chapter ... View full answer

Get step-by-step solutions from verified subject matter experts


