Wastewater containing dissolved hydrogen sulfide (H 2 S) at concentration of 2.50 gmole/m 3 (85 mg/L) enters
Question:
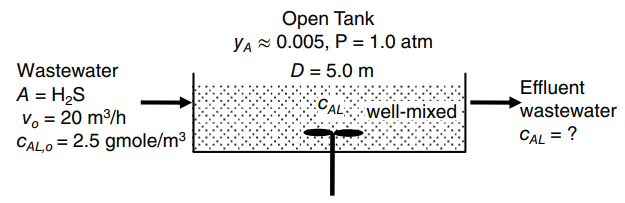
a. Estimate m, the equilibrium distribution constant based upon the mole fraction equilibrium relationship€“e.g., yA,i = mxA,i. Plot out the operating point (xA, yA) and the equilibrium line in mole fraction coordinates. Is the process gas absorption or liquid stripping?
b. What is the overall mass-transfer coefficient Ky based upon the overall gas-phase mole fraction driving force?
c. Perform a material balance on the process. At the conditions of operation, what is the concentration of dissolved H2S exiting the tank, cAL, in units of gmole/m3?
DistributionThe word "distribution" has several meanings in the financial world, most of them pertaining to the payment of assets from a fund, account, or individual security to an investor or beneficiary. Retirement account distributions are among the most...
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Momentum Heat And Mass Transfer
ISBN: 9781118947463
6th Edition
Authors: James Welty, Gregory L. Rorrer, David G. Foster
Question Posted:





