A mass-transfer process is used to remove ammonia (NH 3 , solute A) from a mixture of NH 3 and air, using water as the
a. Prepare an equilibrium distribution plot for NH3 as partial pressure vs. mole fraction (pA €“ xA).
b. What is kx?
c. What are the interface compositions xA,i and pA,i? What are x*A and p*A?
d. What is KG, the overall mass-transfer coefficient based on the gas phase?
e. What is the flux NA for this process?
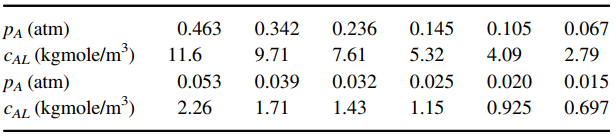
PA (atm) CAL (kgmole/m) PA (atm) CAL (kgmole/m) 0.105 0.463 0.236 0.145 5.32 0.342 9.71 0.067 7.61 4.09 2.79 0.015 0.697 11.6 0.025 0.053 2.26 0.039 0.032 1.43 0.020 1.71 0.925 1.15
Step by Step Solution
3.56 Rating (177 Votes )
There are 3 Steps involved in it
Step: 1
A NH 3 a p A vs x A equilibrium line and operating point b Determine k x c Determi... View full answer

Get step-by-step solutions from verified subject matter experts
100% Satisfaction Guaranteed-or Get a Refund!
Step: 2Unlock detailed examples and clear explanations to master concepts

Step: 3Unlock to practice, ask and learn with real-world examples

See step-by-step solutions with expert insights and AI powered tools for academic success
-
 Access 30 Million+ textbook solutions.
Access 30 Million+ textbook solutions.
-
 Ask unlimited questions from AI Tutors.
Ask unlimited questions from AI Tutors.
-
 Order free textbooks.
Order free textbooks.
-
 100% Satisfaction Guaranteed-or Get a Refund!
100% Satisfaction Guaranteed-or Get a Refund!
Claim Your Hoodie Now!

Study Smart with AI Flashcards
Access a vast library of flashcards, create your own, and experience a game-changing transformation in how you learn and retain knowledge
Explore Flashcards





