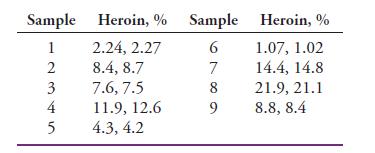
Nine samples of illicit heroin preparations were analyzed in duplicate by a gas chromatographic method. The samples
Question:
Nine samples of illicit heroin preparations were analyzed in duplicate by a gas chromatographic method. The samples can be assumed to have been drawn randomly from the same population. Pool the following data to establish an estimate of σ for the procedure.
Transcribed Image Text:
Sample Heroin, % Sample Heroin, % 2.24, 2.27 8.4, 8.7 7.6, 7.5 11.9, 12.6 4.3, 4.2 1.07, 1.02 14.4, 14.8 1 2 3 21.9, 21.1 4 8.8, 8.4 5 6789
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (10 reviews)

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Analytical Chemistry
ISBN: 9780357450390
10th Edition
Authors: Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch
Question Posted:
Students also viewed these Sciences questions
-
When returns from a project can be assumed to be normally distributed, such as those shown in Figure (represented by a symmetrical, bell-shaped curve), the areas under the curve can be determined...
-
The following data come from independent samples drawn from normally distributed populations. Use these data to test the following hypotheses. Let the Type I error rate be .05. H0: 1 - 2 = 0 Ha: 1 -...
-
The following sample data have been collected from independent samples from two populations. The claim is that the first population median will be larger than the median of the second population. a....
-
Your firm needs to decide how much to ship along each route AND which of the new stores to open. Opening Gamma incurs a fixed cost of $225. Opening Omega incurs a fixed cost of $200. The relevant...
-
For each of the sinking funds, calculate (rounded to the nearest dollar): a. The size of the periodic sinking fund payment. b. The balance in the sinking fund at the time indicated in the last...
-
Do you work hard for your money? Java professionals think they do, reporting long working hours at their jobs. Java developers from around the world were surveyed about the number of hours they work...
-
Corporate sustainability of CPA firms. Corporate sustainability refers to business practices designed around social and environmental considerations. Refer to the Business and Society (March 2011)...
-
The Blazon Manufacturing Companys costing system has two direct- cost categories: direct materials and direct manufacturing labor. Manufacturing overhead (both variable and fixed) is allocated to...
-
Question 38 On January 1, 2018, Irik Corporation issued $1,850,000 face value, 7%, 10-year bonds at $1,725,863. This price resulted in an effective interest rate of 8% on the bonds. The bonds pay...
-
Jane Kent is a licensed CPA. During the first month of operations of her business, the following events and transactions occurred. May 1 Kent invested $25,000 cash. 2 Hired a secretary-receptionist...
-
To test the quality of the work of a commercial laboratory, duplicate analyses of a purified benzoic acid (68.8% C, 4.953% H) sample were requested. It is assumed that the relative standard deviation...
-
Before agreeing to the purchase of a large order of solvent, a company wants to see conclusive evidence that the mean value of a particular impurity is less than 1.0 ppb. What hypotheses should be...
-
Ford Motor Company employed fewer people in 2015 than it did in 1980. Is this decline in employment frictional, structural, cyclical, or some combination of these factors? What information would you...
-
1. Define Image? 2. What is Dynamic Range? 3. Define Brightness? 4. What do you mean by Gray level? 5. What do you mean by Color model? 7. List the hardware oriented color models 8. What is Hue and...
-
11. Define Resolutions 12. What is meant by pixel? 13. Define Digital image 14. What are the steps involved in DIP? 15. What is recognition and Interpretation?
-
16. Specify the elements of DIP system 17. List the categories of digital storage 18. What are the types of light receptors? 19. Differentiate photopic and scotopic vision Photopic vision Scotopic...
-
21. Define subjective brightness and brightness adaptation 22. Define weber ratio 23. What is meant by machband effect? Machband effect means the intensity of the stripes is constant. Therefore it...
-
26. Define sampling and quantization 27. Find the number of bits required to store a 256 X 256 image with 32 gray levels 28. Write the expression to find the number of bits to store a digital image?...
-
During the response to assessed risks step of the overall audit process, the auditor a. Identifies the auditors own risks from accepting the engagement. b. Develops the audit plan and detailed audit...
-
Read the following description and Write a response of it. The discretion of public administrators can be decreased, but not altogether eliminated. Officials will use their discretion in any given...
-
Silver ion is being considered for separating I- from SCN2 in a solution that is 0.040 M in KI and 0.080 M in NaSCN. (a) What Ag1 concentration is needed to lower the I2 concentration to 1.0 10-6 M?...
-
What mass of AgBr dissolves in 200 mL of 0.200 M NaCN? Ag+ + 2CN--Ag(CN), 2 = 1.3 1021
-
Why are simplifying assumptions restricted to relationships that are sums or differences?
-
September 1 . Purchased a new truck for $ 8 3 , 0 0 0 , paying cash. September 4 . Sold the truck purchased January 9 , Year 2 , for $ 5 3 , 6 0 0 . ( Record depreciation to date for Year 3 for the...
-
Find the NPV for the following project if the firm's WACC is 8%. Make sure to include the negative in your answer if you calculate a negative. it DOES matter for NPV answers
-
What is the value of a 10-year, $1,000 par value bond with a 12% annual coupon if its required return is 11%?

Study smarter with the SolutionInn App


