Suggest a range of sample masses for the indicated primary standard if it is desired to use
Question:
Suggest a range of sample masses for the indicated primary standard if it is desired to use between 35 and 45 mL of titrant:
(a) 0.180 M HClO4 titrated against Na2CO3(CO2 product).
(b) 0.102 M HCl titrated against Na2C2O4.
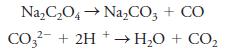
(c) 0.180 M NaOH titrated against benzoic acid.
(d) 0.090 M Ba(OH)2 titrated against KH(IO3)2.
(e) 0.065 M HClO4 titrated against TRIS.
(f) 0.060 M H2SO4 titrated against Na2B4O7 . 10H2O. Reaction:
![]()
Transcribed Image Text:
Na,C,O4→ Na,Co, + CO CO?- + 2H + → H2O + CO,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 30% (10 reviews)
a A suitable ...View the full answer

Answered By

Brian Otieno
I'm Brian , an experienced professional freelancer with countless hours of success in freelancing many subjects in different disciplines. Specifically, I have handled many subjects and excelled in many disciplines. I have worked on many Computer Science projects and have been able to achieve a lot in that field. Additionally, I have handled other disciplines like History, Humanities, Social Sciences, Political science, Health care and life science, and Religion / Theology. My experience generally in these subjects has made me able to deliver high-quality projects in a very timely fashion. I am very reliable at my job and will get the work done in time, no matter what. In Addition, I have managed to ensure that the work meets my client's expectations and does not cause an error. I am a hard-working and diligent person who is highly responsible for everything I do. Generally, Freelancing has made me more accountable for doing my job. Additionally, I have had a passion for writing for the last seven years in this field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Analytical Chemistry
ISBN: 9780357450390
10th Edition
Authors: Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch
Question Posted:
Students also viewed these Sciences questions
-
It is desired to use a 0.75-m diameter beach ball to stop a drain in a swimming pool. Obtain an expression that relates the drain diameter D and the minimum water depth h for which the ball will...
-
It is desired to simulate flow past a ridge or bump by using a streamline above the flow over a cylinder, as shown in Fig. P8.50. The bump is to be a/2 high, as shown. What is the proper elevation h...
-
It is desired to produce an aligned and continuous fiber-reinforced epoxy composite having a maximum of 50 vol% fibers. In addition, a minimum longitudinal modulus of elasticity of 50 GPa (7.3 106...
-
The IQ and VIQ are tools insurance producers use to learn more about internal assumptions regarding factors that ultimately control policy performance. They have information in all of the following...
-
This problem demonstrates the dependence of an annuitys present value on the size of the periodic payment. Calculate the present value of 25 end-of-year payments of: a. $1000. b. $2000. c. $3000. Use...
-
The average early-bird special admission price for a movie is $5.81. If the distribution of movie admission charges is approximately normal with a standard deviation of $0.81, what is the probability...
-
4. In a business combination, the additional price paid over the fair value of net assets acquired is: a Recorded as a gain from a bargain purchase b Amortized according to its useful life c Subject...
-
A business executive is offered a management job at Generous Electric Company, which offers him a 5-year contract that calls for a salary of $62,000 per year, plus 600 shares of GE stock at the end...
-
$10.65 9.00 1.60 Direct materials Direct labor Variable overhead Fixed overhead ($3.40 general company overhead, $1.90 depreciation, and, $0.70 supervision) Total cost per drum 6.00 $ 27.25 A...
-
A machine that is currently used in manufacturing circuit board card locks has AW = $-63,000 per year. A possible replacement is under consideration with a first cost of $64,000 and an operating cost...
-
A 25.0-mL aliquot of vinegar was diluted to 250 mL in a volumetric flask. Titration of 50.0-mL aliquots of the diluted solution required an average of 25.23 mL of 0.09041 M NaOH. Express the acidity...
-
The benzoic acid extracted from 86.7 g of catsup required a 15.61-mL titration with 0.0654 M NaOH. Express the results of this analysis in terms of percent sodium benzoate (144.10 g/mol).
-
Most metals are shiny, that is, they reflect light. How does the band theory for metals explain this characteristic?
-
How much Group Revenue do you currently have booked for September 2024? (format $, no decimals; e.g, $5,000) How much additional Group Revenue do you need to book to hit your budget for September...
-
What strategies do businesses have in place that promote equal opportunity within the organization? Do most businesses offer career development and training to its employees? If so, why? How do...
-
How do you do the step method in cost accounting? I need help understanding the difference between the step method and reciprocal method.
-
1- How do long-term care changing the health care system in the 21st century? What ethical issues do you see playing a role in this situation? 2- Why do long-term health care and palliative care...
-
How do the processes and layouts of Harley Davidson enable it to deliver goods and services to its customer? (How do they do what they do with the resources they have?)
-
Fortune Software Corp. has assembled the following data for the years ending December 31, 2018 and 2017: Requirement 1. Prepare Fortunes statement of cash flows using the indirect method to report...
-
Tanaka Company's cost and production data for two recent months included the following: March April Production (units).........300................600 Rent.....................$1,800............$1,800...
-
Calculate the 95% confidence interval for each set of data in Problem 7-4 if s is a good estimate of s and has a value of Set A, 0.30; Set C, 0.070; Set E, 0.0090
-
An atomic absorption method for the determination of the amount of iron present in used jet engine oil was found from pooling 30 triplicate analyses to have a standard deviation s = 3.6 mg Fe/mL. If...
-
How many replicate measurements are needed to decrease the 95 and 99% confidence limits for the analysis described in Problem 7-7 to 2.2 mg Fe / mL?
-
why would an auditor want to complete dual-purpose tests? what procedure can be put into place to help prevent fraud? List 4 procedures.
-
Based on the following information, calculate sustainable growth rate for Groot, Inc.: Profit margin= 7.1% Total asset turnover = 1.90 Total debt ratio = .45 Payout ratio = 20% What is the ROA here?
-
Consider the following: a call option on a stock has strike price $100, premium of $5 and the current price of the underlying stock is $100. If you buy the call option today, what is your holding...

Study smarter with the SolutionInn App


