Rank the following compounds in order of increasing oxidation state. OH HC-CH-CHOH A -OOC-CH-COO- B HCCH,CH3 HC-CH=CH
Question:
Rank the following compounds in order of increasing oxidation state.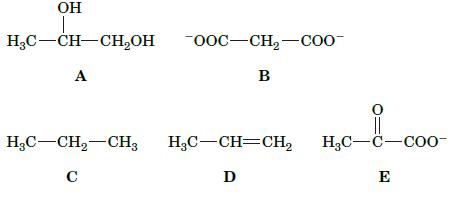
Transcribed Image Text:
OH HC-CH-CHOH A -OOC-CH-COO- B HCCH,CH3 HC-CH=CH HC-C-COO- C D E
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
To rank the compounds in order of increasing oxidation state we need to consider the changes in the oxidation state of carbon in each compound The oxi...View the full answer

Answered By

Abigael martinez
I have been a tutor for over 3 years and have had the opportunity to work with students of all ages and backgrounds. I have a strong belief that all students have the ability to learn and succeed if given the right tools and support. I am patient and adaptable, and I take the time to get to know each student's individual learning style in order to best support their needs. I am confident in my ability to help students improve their grades and reach their academic goals.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Biochemistry Life At The Molecular Level
ISBN: 9781118918401
5th Edition
Authors: Donald Voet, Judith G Voet, Charlotte W Pratt
Question Posted:
Students also viewed these Sciences questions
-
Rank the following compounds in order of increasing λ max :
-
Rank the following compounds in order of increasing stability based on relative ring strain.
-
Rank the following compounds in order of increasing amount of hydrate present at equilibrium. Br Br CHO CHO
-
Suppose treacle is an array of 10 floats. Declare a pointer that points to the first element of treacle and use the pointer to display the first and last elements of the array.
-
Parker Packaging Company manufactures cardboard and packaging products, with two operating divisions, the Cardboard and Packaging Divisions. Condensed divisional income statements, which involve no...
-
Use the DuPont equation to show how working capital policy affects a firms expected ROE. AppendixL01
-
How can the reasonableness of the estimated required holding period return be evaluated?
-
The following data pertain to British Isles Aggregates Company, a producer of sand, gravel, and cement, for the year just ended. Sales...
-
7 Assume that a company is planning to invest $150,000 in a project that will last three years. The project will produce the following net cash inflows: Year 1 Year 2 Year 3 $ 65,000 $ 75,000 $? 2...
-
A strain of bacteria isolated from an alkaline lake with a high concentration of arsenic is able to incorporate As into biological molecules. What class of molecules is most likely to contain As as...
-
Explain why a heterotrophic organism may require vitamins, whereas an autotroph does not.
-
Explain the connection between short-circuit Boolean expressions and normal-order evaluation. Why is cond a special form in Scheme, rather than a function?
-
In 2024, the Westgate Construction Company entered into a contract to construct a road for Santa Clara County for $10,000,000. The road was completed in 2026. Information related to the contract is...
-
Briefly describe the case you have chosen. Categorize the social worker's experience as vicarious trauma, compassion fatigue, or burnout. Provide justification. Identify the social worker's score on...
-
Given f(x) below, find f'(x). f(x) = = m 5z In (2) et dt
-
Olsen & Alain, CPAs (O&A) performed the audit of Rocky Point Brewery (RPB), a public company in 20X1 and 20X2. In 20X2, O&A also performed tax services for the company. Which statement best describes...
-
Exercise 9-4 (Algo) Prepare a Flexible Budget Performance Report [LO9-4] Vulcan Flyovers offers scenic overflights of Mount Saint Helens, the volcano in Washington State that explosively erupted in...
-
Given the initial conditions, y(0) = 1 and y'(0) = 0, solve the following initial-value problem from t = 0 to 4: d2y/dt2 + 4y = 0 Obtain your solutions with (a) Euler's method and (b) The fourthorder...
-
Medi-Exam Health Services, Inc. (MEHS), located in a major metropolitan area, provides annual physical screening examinations, including a routine physical, EKG, and blood and urine tests. MEUS's...
-
Some bacteria catabolize glucose by the Entner-Doudoroff pathway, a variant of glycolysis in which glucose-6-phosphate is converted to 6-phosphogluconate (as in the pentose phosphate pathway) and...
-
The aldolase reaction can proceed in reverse as an enzymatic aldol condensation. If the enzyme were not stereospecific, how many different products would be obtained?
-
Bacterial aldolase does not form a Schiff base with the substrate. Instead, it has a divalent Zn2+ ion in the active site. How does the ion facilitate the aldolase reaction?
-
Comfort Golf Products is considering whether to upgrade its equipment Managers are considering two options. Equipment manufactured by Stenback Inc. costs $1,000,000 and will last five years and have...
-
Weaver Corporation had the following stock issued and outstanding at January 1, Year 1: 71,000 shares of $10 par common stock. 8,500 shares of $60 par, 6 percent, noncumulative preferred stock. On...
-
Read the following case and then answer questions On 1 January 2016 a company purchased a machine at a cost of $3,000. Its useful life is estimated to be 10 years and then it has a residual value of...

Study smarter with the SolutionInn App


