10 mol/s of gas flow through a turbine. Find the change in enthalpy that the gas experiences:...
Question:
10 mol/s of gas flow through a turbine. Find the change in enthalpy that the gas experiences:
A. The gas is steam, with an inlet temperature and pressure T = 600°C and P = 10 bar, and an outlet temperature and pressure T = 400°C and P = 1 bar. Use the steam tables.
B. The gas is steam, with the same inlet and outlet conditions as in part A. Model the steam as an ideal gas using the value of C*P given in Appendix D.
C. The gas is nitrogen, with an inlet temperature and pressure of T = 300 K and P = 10 bar, and an outlet temperature and pressure T = 200 K and P = 1 bar. Use Figure 2-3.
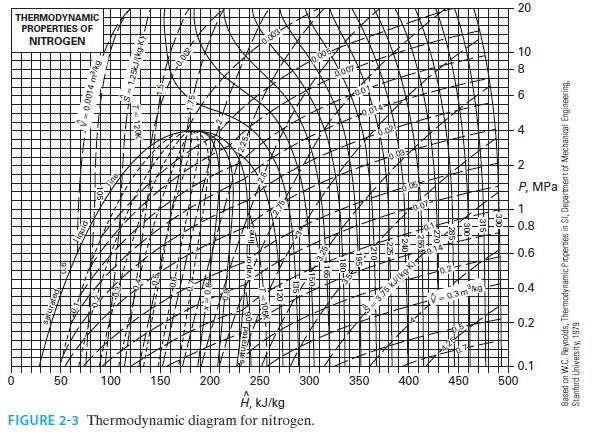
D. The gas is nitrogen with the same inlet and outlet conditions as in part C. Model the nitrogen as an ideal gas using the value of C*P given in Appendix D.
E. Compare the answers to parts A and B and to parts C and D. Comment on whether they are significantly different from each other, and if so, why.
Step by Step Answer:

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco





