Question
10 mol/s of gas flow through a turbine. Find the change in enthalpy that the gas experiences: A. The gas is steam, with an inlet
10 mol/s of gas flow through a turbine. Find the change in enthalpy that the gas experiences:
A. The gas is steam, with an inlet temperature and pressure T = 600°C and P = 10 bar, and an outlet temperature and pressure T = 400°C and P = 1 bar. Use the steam tables.
B. The gas is steam, with the same inlet and outlet conditions as in part A. Model the steam as an ideal gas using the value of ![]() given in Appendix D.
given in Appendix D.
C. The gas is nitrogen, with an inlet temperature and pressure of T = 300 K and P = 10 bar, and an outlet temperature and pressure T = 200 K and P = 1 bar. Use Figure.
Figure Thermodynamic diagram for nitrogen.
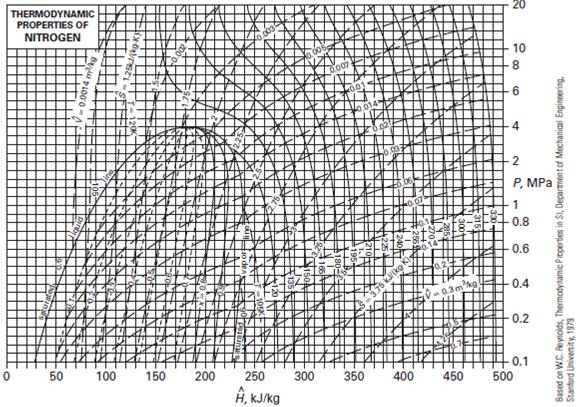
D. The gas is nitrogen with the same inlet and outlet conditions as in part C. Model the nitrogen as an ideal gas using the value of ![]() given in Appendix D.
given in Appendix D.
E. Compare the answers to parts A and B and to parts C and D. Comment on whether they are significantly different from each other, and if so, why.
THERMODYNAMIC PROPERTIES OF NITROGEN 0 50 100 150 0.002 200 250 , kJ/kg 300 2007 350 400 0-0.3mkg 450 10 8 6 4 -2 P, MPa 0.6 0.4 0.2 -0.1 500 Based on W.C. Reynolds, Thermodynamic Properties in SI, Department of Mechanical Engineering. Stanford University, 1979
Step by Step Solution
3.42 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


