100 kg/min of liquid nitrogen is produced by the steady-state process shown in Figure 5-17. 1. Nitrogen...
Question:
100 kg/min of liquid nitrogen is produced by the steady-state process shown in Figure 5-17.
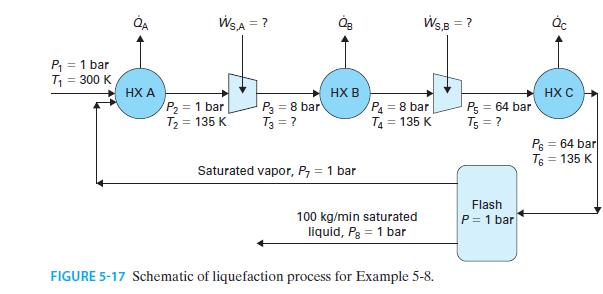
1. Nitrogen enters the process at P = 1 bar and T = 300 K.
2. The nitrogen is cooled in a heat exchanger (A) to T = 135 K.
3. The nitrogen is compressed from P = 1 bar to P = 8 bar in one compressor (A), and from P = 8 bar to P = 64 bar in a second compressor (B). Each compression step is followed by a heat exchanger that cools the nitrogen to T = 135 K.
4. The nitrogen at P = 64 bar and T = 135 K enters an adiabatic flash vessel in which P = 1 bar.
5. The liquid leaving the vessel is removed as product: saturated liquid nitrogen at P = 1 bar.
6. The vapor leaving the vessel is recycled to the heat exchanger described in step 2, where it mixes with the fresh nitrogen feed. Assuming both compressors have an efficiency of 80% and no pressure drop occurs in any heat exchanger, find the total rate at which work is added to each of the two compressors. How much work is required for each kilogram of liquid nitrogen produced?
Step by Step Answer:

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco





