A mixture that contains 40% by mole n-heptane in n-decane is to be separated in a series
Question:
A mixture that contains 40% by mole n-heptane in n-decane is to be separated in a series of flush separators until a stream is obtained that contains at least 95% n-heptane. Determine the number of separators needed, their temperature, and the recovery of n-heptane if all separators are at 1.013 bar and V/L = 3 in all separators. Txy data are given below:
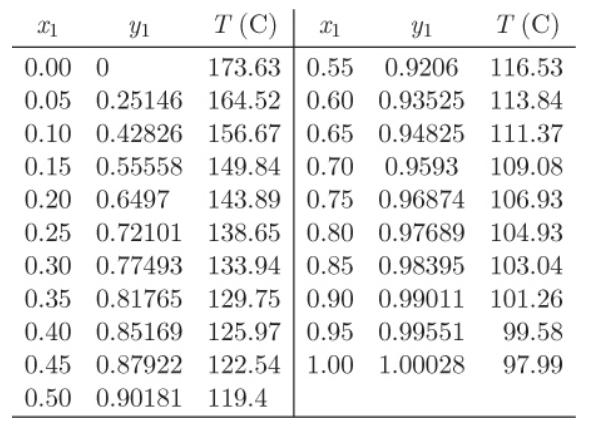
Transcribed Image Text:
X1 T (C) X1 Yı T (C) 0.00 0 173.63 0.55 0.9206 116.53 0.05 0.25146 164.52 0.60 0.93525 113.84 0.10 0.42826 156.67 0.65 0.94825 111.37 0.15 0.55558 149.84 0.70 0.9593 109.08 0.20 0.6497 143.89 0.75 0.96874 106.93 0.25 0.72101 138.65 0.80 0.97689 104.93 0.30 0.77493 133.94 0.85 0.98395 0.35 0.81765 129.75 0.90 0.99011 0.40 0.85169 125.97 0.95 0.99551 0.45 0.87922 122.54 1.00 103.04 101.26 1.00028 0.50 0.90181 119.4 Y1 99.58 97.99
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (8 reviews)
To determine the number of separators needed their temperature and the recovery of nheptane in each separator we can use the concept of flash distilla...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9780132693066
1st Edition
Authors: Themis Matsoukas
Question Posted:
Students also viewed these Engineering questions
-
A steady stream of equimolar N2 and CO2 mixture at 100 kPa and 18C is to be separated into N2 and CO2 gases at 100 kPa and 18C. Determine the minimum work required per unit mass of mixture to...
-
Journalize the 10 transactions for your company using excel (must include descriptions). Your opening entry should include the following 4 items: Bank $10,000 Supplies $5,000 Bank Loan $20,000...
-
A total of 2,000 gallons of 70 wt% ethanol in water, having a specific gravity of 0.871, is to be separated at 1 atm in a batch rectifier operating at constant distillate composition with a constant...
-
What is a buy-sell agreement, and how does life insurance facilitate it?
-
The government places a tax on the purchase of socks. a. Illustrate the effect of this tax on equilibrium price and quantity in the socks market. Identify the following areas both before and after...
-
Lucatelli Pasta Company prepares, packages, and distributes six frozen pasta entrees in two different Gc Driver Rates container sizes. It prepares the different pastas and different sizes in large...
-
In a build operate transfer agreement how does the business that built the facility ensure that they profit from the agreement? LO.1
-
Ross Corporation is a debtor in a reorganization proceeding under Chapter 11 of the Bankruptcy Code. By fair and proper valuation, its assets are worth $100,000. The indebtedness of the corporation...
-
House Corporation has been operating profitably since its creation in 1960. At the beginning of 2019. House acquired a 70 percent ownership in Wilson Company. At the acquisition date, House prepared...
-
A stream that contains a mixture of methane (25% by mol) and carbon monoxide is compressed from 1 bar, 35 to 12 bar. The compressor efficiency is 90%. Treating the mixture as an ideal gas, calculate...
-
Use the data below for the system ethyl propyl ether (1)-chloroform (2) to answer the following questions: a) What is the boiling point of chloroform at 0.5 bar? b) Is this a maximum boiling or...
-
(a) Calculate the pH of 1.89 * 10 5 m and 9.64 * 10 7 m HClO(aq), ignoring the effect of the autoprotolysis of water. (b) Repeat the calculations, taking into account the autoprotolysis of water.
-
A baseball player's slugging percentage SLG can be calculated with the following formula (which is an example of a rational function): SLG = H+2B+2x(3B)+3x(HR) AB Q Image transcription text H+2B+2x...
-
Question During 2021, Cassandra Albright, who is single, worked part-time at a doctor's office and received a W-2. She also had a cash-basis consulting practice that had the following income and...
-
Shelly Beaman (social security number 412-34-5670) Is single and resides at 540 Front Street, Ashland, NC 27898. Shelly's W-2 wages Federal withholding Social security wages Social security...
-
P14-26. Forecasting with Parsimonious Method and Estimating Share Value Using the ROPI Model Following are income statements and balance sheets for Cisco Systems. CISCO SYSTEMS Consolidated...
-
A little lesson on horseracing.An exacta wager is where you pick the horse that you think will come first, and another who will come second. A trifecta wager is where you pick 3 horses that you think...
-
Search the Internet for at least two recently published MD&As. Print the documents and perform the following: a. Compare and contrast the content and style of the two MD&As. b. Summarize your...
-
H.J. Heinzs annual dividends were as follows: 1990 ..............$0.540 1991.............. 0.620 1992 .............. 0.700 1993.............. 0.780 1994 .............. 0.860 1995 .............. 0.940...
-
Several processes are described. Indicate whether each is reasonably modeled as reversible, and if not, indicate what aspect of the process makes it irreversible. A . The inside of a refrigerator is...
-
A steady-state distillation column is designed to separate benzene from toluene. The separation is nearly enough complete that, for the purposes of designing the reboiler and the condenser, we can...
-
A liquid stream contains 1 lb m /s of the compound at T = 100F and P = 1 atm. It needs to be boiled and heated to P = 1 atm and 175F, as that is the temperature at which it must enter a chemical...
-
Series of Compound Interest Techniques The following are several situations involving compound interest. Required: Using the appropriate table, solve each of the following: ( Click here to access the...
-
If Clark Kelly has recognized gain on an exchange of like-kind property held for investment use, where does Clark report the gain? First on Form 8824, then carried to Schedule D. First on Form 8824,...
-
An investor put 40% of her money in Stock A and 60% in Stock B. Stock A has a beta of 1.2 and Stock B has a beta of 1.6. If the risk-free rate is 5% and the expected return on the market is 12%,...

Study smarter with the SolutionInn App


