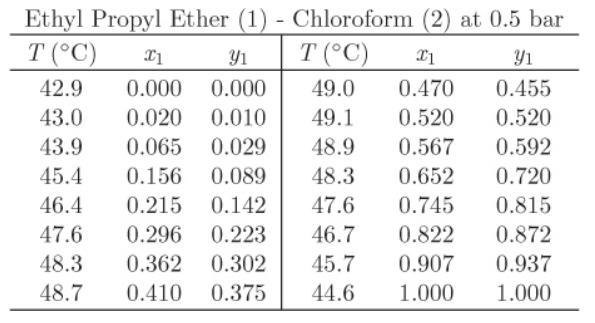
Use the data below for the system ethyl propyl ether (1)-chloroform (2) to answer the following questions:
Question:
Use the data below for the system ethyl propyl ether (1)-chloroform (2) to answer the following questions:
a) What is the boiling point of chloroform at 0.5 bar?
b) Is this a maximum boiling or minimum boiling azeotrope?
c) What is the composition at the azeotropic point?
d) A mixture of the two components contains 80% by mol ethyl propyl ether. What is the phase of this mixture at 48.3 °C, 0.5 bar? If a two-phase system, report the composition of the two phases and their relative amounts.
e) One mol of a solution, whose bubble point at 0.5 bar is 48.3 °C, is mixed with chloroform until the final mixture contains 50% chloroform (by mol). How much chloroform is needed?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9780132693066
1st Edition
Authors: Themis Matsoukas
Question Posted:





