As an undergraduate chemical engineering student, you are involved in a summer research project that requires
Question:
As an undergraduate chemical engineering student, you are involved in a summer research project that requires you to measure the molar volume of a methanol (1) + water (2) binary system at 298.15 K along an isobar. You measure the following data, which is reported in Table 9-5 as
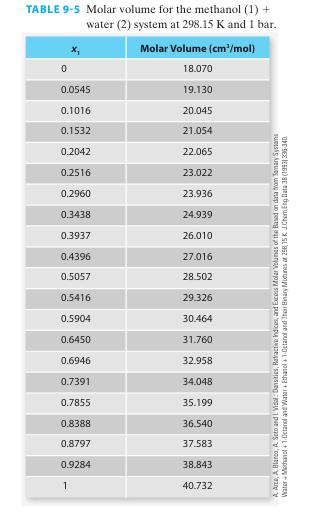
A month later, after you have finished the project and have left the facility, you get a message from your research advisor asking you to estimate (as accurately as you can) the molar volume of the methanol + water mixture with 75% by mole methanol at the same T and P of your experiment. What molar volume will you report? What are the partial molar volumes of each component at this state?
Step by Step Answer:

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco





