Compound A is an industrial solvent and is widely enough used that extensive data on it is
Question:
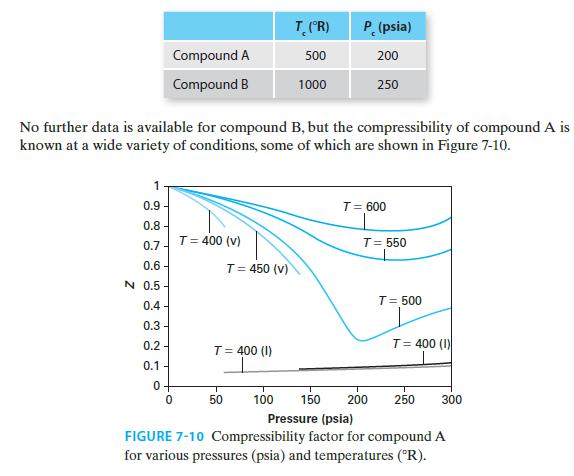
Compound A is an industrial solvent and is widely enough used that extensive data on it is available. Compound B is a recently invented compound that can serve the same purpose as compound A, and we now wish to determine whether compound B can be manufactured more cheaply than compound A. We have completed a flowsheet for the manufacturing process, but in order to design and size the needed equipment, we must be able to estimate the molar volume of compound B at a variety of temperatures and pressures. Here we will estimate the molar volumes of compound B:
A. In the liquid phase at T = 900°R and P = 200 psia
B. In the vapor phase at T = 900°R and P = 50 psia
C. In the supercritical state at T = 11008R and P = 300 psia
The critical points for both compounds are given here.
Step by Step Answer:

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco





