Estimate the partial molar enthalpy of sulfuric acid at 140F at the following two compositions using Figure
Question:
Estimate the partial molar enthalpy of sulfuric acid at 140°F at the following two compositions using Figure P9-22.
A. 30% by wt sulfuric acid
B. 80% by wt sulfuric acid
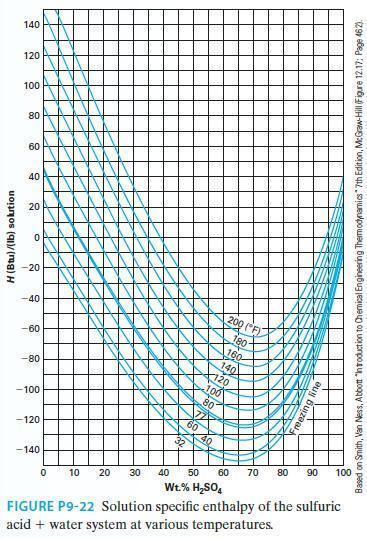
Transcribed Image Text:
H (Btu)/(lb) solution 140 120 100 80 60 40 20 O -20 -40 -60 -80 -100 -120 -140 } 200 (F) 180- 190 K But Bu 0 10 20 30 40 50 60 70 80 Wt.% H₂SO4 FIGURE P9-22 Solution specific enthalpy of the sulfuric acid + water system at various temperatures. 90 100 Based on Smith, Van Ness, Abbott "introduction to Chemical Engineering Thermodynamics" 7th Edition, McGraw-Hill (Figure 12.17: Page 462).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (8 reviews)

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:
Students also viewed these Engineering questions
-
Using tabulated experimental data from the literature for either the excess molar volume or excess molar enthalpy of a system of your choice, provide the following information. A. A plot of the...
-
The molar enthalpy of a mixture of hydrogen fluoride (1) and water (2) at 20C is given as (Tyner, 1949) H = -1850 - 28240x, +26800x where II[=] J/mol Calculate the partial molar enthalpy of water in...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
In Problems 1130, solve each equation by factoring. x 2 - 9x = 0
-
Dicks Sporting Goods is a chain of full-line sporting goods retail stores offering a broad assortment of brand name sporting goods equipment, apparel, and footwear. Dicks Sporting Goods had its...
-
Consumer Reports uses a survey of readers to obtain customer satisfaction ratings for the nations largest retailers (Consumer Reports, March 2012). Each survey respondent is asked to rate a specified...
-
Why are accruals called spontaneous sources of funds, what are their costs, and why dont firms use more of them? AppendixL01
-
CVP, sensitivity analysis Technology of the Past (TOP) produces old-fashioned simple corkscrews. Last year was not a good year for sales but TOP expects the market to pick up this year. Last years...
-
Holly Company has the following information for December 1 to December 31. All direct materials are 100% complete. Work-in-Process Beginning balance December 300 units, 20% complete for conversion $...
-
Calculate the van Laar parameters, L 12 and L 21 , for the benzene (1) + toluene (2) system at 25C through use of the van der Waals equation of state, What would be the value for the activity...
-
A mass of 500 lb m of 40 wt% sulfuric acid solution at 140F is diluted with 200 lb m of pure water at 100F. What is the concentration of the resulting solution? What is the heat effect (liberated or...
-
Determine the overall efficiency for the column designed by the ratebased method in Aspen Plus Lab 13, step 10. If you saved your program, use the saved program as the starting point for the...
-
How do social identity processes, such as categorization, identification, and comparison, influence team cohesion and performance within complex organizational environments ?
-
How do calculate sales forecast and expense forecast for several years
-
In your opinion, do you feel the Six Pillars apply primarily to business-to-consumer marketing, business-to-business marketing, or both?
-
Place yourself in context; You're working, trying to do well, running into all sorts of organizational constraints. First, describe a scenario to illustrate one of the eight organizational...
-
what ways do mindfulness-based stress reduction (MBSR) techniques contribute to reducing stress, and what are their limitations ?
-
When deuterated phenanthrene oxide undergoes an epoxide rearrangement in water, 81% of the deuterium is retained in the product. a. What percentage of the deuterium will be retained if an NIH shift...
-
When the Department of Homeland Security created a color-coded system to prepare government officials and the public against terrorist attacks, what did it do right and what did it do wrong?
-
Starting with the fully protonated species, write the stepwise acid dissociation reactions of the amino acids glutamic acid and tyrosine. Be sure to remove the protons in the correct order. Which...
-
Draw the structure of the predominant form of pyridoxal-5-phosphate at pH 7.00.
-
What fraction of ethane-1,2-dithiol is in each form (H 2 A, HA + , A 2+ ) at pH 8.00? at pH 10.00?
-
Northwood Company manufactures basketballs. The company has a ball that sells for $25. At present, the ball is manufactured in a small plant that relies heavily on direct labor workers. Thus,...
-
14. Post the following November transactions to T-accounts for Accounts Payable and Inventory, indicating the ending balance (assume no beginning balances in these accounts). A. purchased merchandise...
-
Question 30 Not yet answered Marked out of 1.00 p Flag question Adjusting, correcting, and closing entries are normally recorded in the General Journal Cash Receipts Journal Cash Disbursement Journal...

Study smarter with the SolutionInn App


