Find the molar volume of methane at P = 15 bar and T = 200C (it is
Question:
Find the molar volume of methane at P = 15 bar and T = 200°C (it is a gas at these conditions), using the following methods.
A. The Soave equation of state
B. The Peng-Robinson equation of state
C. The virial equation of state
D. The Lee-Kesler generalized correlation
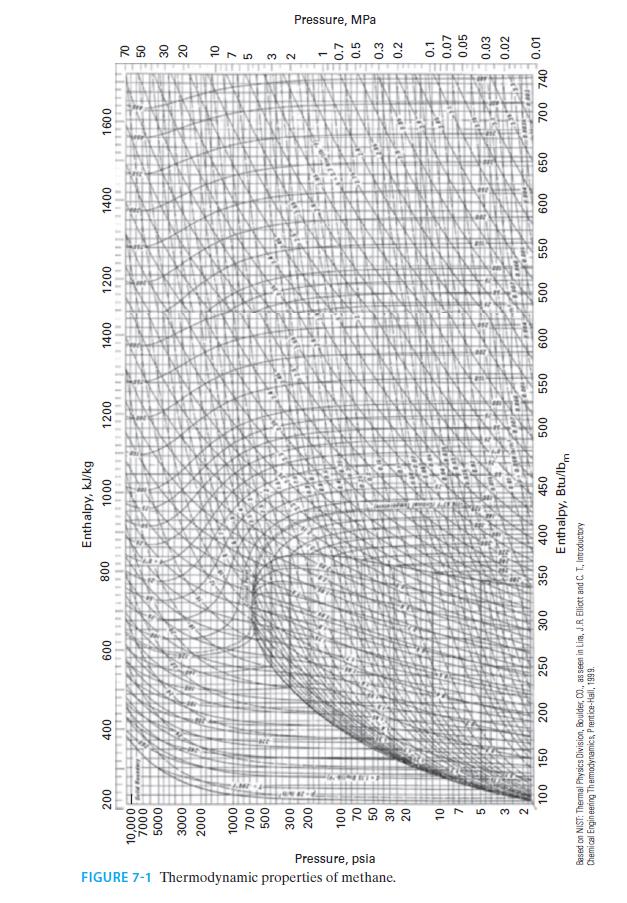
E. Figure 7-1
Transcribed Image Text:
FIGURE 7-1 Thermodynamic properties of methane. Pressure, psia 200 10,000 7000 5000 3000 2000 1000 700 500 300 200 100 70 50 30 20 10 7 5 feld Founday 400 600 800 3 2 100 150 200 250 300 350 Enthalpy, kJ/kg 1000 400 450 Enthalpy, Btu/lbm Based on NIST: Thermal Physics Division, Boulder, 00., as seen in Lima, J.R. Elliott and C. T.. Introductory Chemical Engineering Thermodynamics, Prentice-Hall, 1999. 1200 500 1400 1200 111 550 600 500 1400 550 600 650 1600 700 740 70 50 30 20 27532 10 1 0.7 0.5 0.3 0.2 0.1 0.07 0.05 0.03 0.02 0.01 Pressure, MPa
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
A The Soave equation of state The Soave equation of state is a cubic equation of state that is more accurate than the ideal gas law at high pressures ...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:
Students also viewed these Engineering questions
-
Find the molar volume of nitrogen at P = 10 bar and T = 330 K, using the following methods. A. The Soave equation of state B. The Peng-Robinson equation of state C. The virial equation of state D....
-
Question 3. Find the second derivative of y = x - 1 Question 4. If f(x) = x-1 Find f'(-2).
-
Find the molar volume of Freon 22 at P = 5 bar and T = 20C (it is a vapor at these conditions), using the following methods. A. The Soave equation of state B. The Peng-Robinson equation of state C....
-
Which of the following is not a strategic disadvantage of vertical integration? Vertical integration poses all kinds of capacity-matching problems (achieving the most efficient scale of operation for...
-
Refer to Exhibit 10-7 which presents the methodology for analyzing exchange rate variances. Describe in your own words what this methodology accomplishes.
-
Referring to Problem 13.60, instead of predicting the unit density, you now wish to predict the foam diameter from results stored in PackagingFoam4 . Develop a multiple regression model that uses die...
-
Luminant Productions Limited produces light fittings from a small factory unit. The company's directors have just met to discuss the sales budget and related matters for the next quarter, and have...
-
The Chang Company is considering the purchase of a new machine to replace an obsolete one. The machine being used for the operation has a book value and a market value of zero. However, the machine...
-
Hillside issues $ 2 , 6 0 0 , 0 0 0 of 5 % , 1 5 - year bonds dated January 1 , 2 0 2 1 , that pay interest semiannually on June 3 0 and December 3 1 . Problem 1 0 - 1 A ( Algo ) Straight - Line:...
-
Use the Joback method to estimate T c and P c of each of the following compounds. A. Butane B. 1-Hexanol C. 2-chloropentane D. 3-Hexene E. 1,3-butadiene
-
Using data in Appendix C-1, determine the Peng-Robinson parameters a and b for each of the following compounds at the temperature T = 100C. A. Ethane B. Acetone C. Benzene D. Toluene E. Decane Name...
-
Given the following hypotheses: H0: 20 H1: < 20 A random sample of five resulted in the following values: 18, 15, 12, 19, and 21. Assume a normal population. Using the .01 significance level, can...
-
Following is a partially completed balance sheet for Epsico Incorporated at December 31, 2022, together with comparative data for the year ended December 31, 2021. From the statement of cash flows...
-
Solve the following linear system by Gaussian elimination with back-substitution without introducing fractions in your row-reduction. If there is no solution, explain why. -3x+8y + 82 = -8 -2x+ y -...
-
Introduction Some predictions are a slam dunk. Retail will continue to be driven by technology. Science fiction is coming to life in the form of robotics and virtual reality. And the Internet will...
-
Oswego Clay Pipe Company provides services of $ 5 0 , 0 0 0 to Southeast Water District # 4 5 on April 1 2 of the current year with terms 1 / 1 5 , n / 6 0 . What would Oswego record on April 1 2 ?...
-
Assume the following excerpts from a company's balance sheet: Property, plant, and equipment Beginning Balance $3,500,000 Ending Balance $3,700,000 $1,100,000 $800,000 Long-term investments During...
-
A cube that is 4.00 cm on a side and of density 8.00 102 kg/m3 is attached to one end of a spring. The other end of the spring is attached to the base of a beaker. When the beaker is filled with...
-
Suppose the index goes to 18 percent in year 5. What is the effective cost of the unrestricted ARM?
-
The following data are the measured temperature T of water owing from a hot water faucet after it is turned on at time t = 0. a. Plot the data, connecting them rst with straight lines and then with a...
-
a. Solve the following matrix equation for the matrix C. A(BC + A) = B b. Evaluate the solution obtained in part a for the case 7 9 4 -3 A = -2 4. B = 7 6 ]
-
Solve the following problems using matrix inversion. Check your solutions by computing A -1 A. a. b. c. d. 2 + %3D 5 = 5 9 3 7
-
The Purple Face Mask Company is carefully examining its pricing based upon a change in product demand. Currently, the company uses a volume-based pricing system with direct labor hours as the cost...
-
Golden Manufacturing Company started operations by acquiring $150,000 cash from the issue of common stock. On January 1, Year 1, the company purchased equipment that cost $120,000 cash, had an...
-
On July 1, 2021, Empire Incorporated lends $8,400 to a customer and receives a 10% note due in two years. Interest is due in full on July 1, 2023, the due date of the note. What is the amount of...

Study smarter with the SolutionInn App


