In a process at your plant, you are mixing two liquids: benzene (1) and 2-propanol (2). You
Question:
In a process at your plant, you are mixing two liquids: benzene (1) and 2-propanol (2).
You would like to create a molar enthalpy vs. composition diagram for this mixture and have found molar enthalpy of mixing data available in Table 9-3.
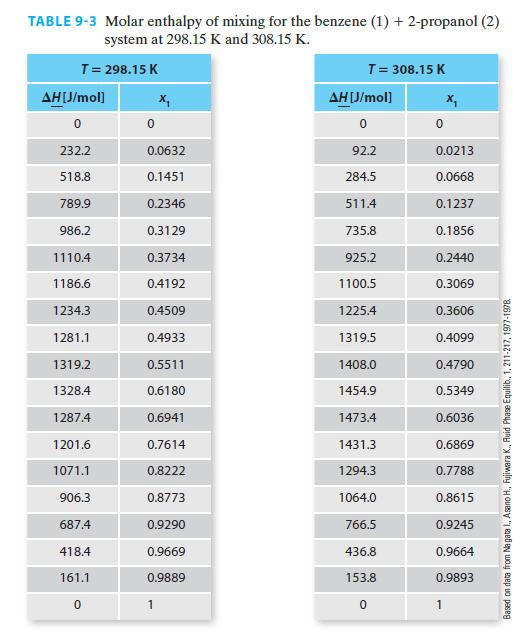
Transcribed Image Text:
TABLE 9-3 Molar enthalpy of mixing for the benzene (1) + 2-propanol (2) system at 298.15 K and 308.15 K. T = 298.15 K AH[J/mol] 232.2 518.8 789.9 986.2 1110.4 1186.6 1234.3 1281.1 1319.2 1328.4 1287.4 1201.6 1071.1 906.3 687.4 418.4 161.1 0 0.0632 0.1451 0.2346 0.3129 0.3734 0.4192 0.4509 0.4933 0.5511 0.6180 0.6941 0.7614 0.8222 0.8773 0.9290 0.9669 0.9889 1 T = 308.15 K AH [J/mol] 0 92.2 284.5 511.4 735.8 925.2 1100.5 1225.4 1319.5 1408.0 1454.9 1473.4 1431.3 1294.3 1064.0 766.5 436.8 153.8 0 0 0.0213 0.0668 0.1237 0.1856 0.2440 0.3069 0.3606 0.4099 0.4790 0.5349 0.6036 0.6869 0.7788 0.8615 0.9245 0.9664 0.9893 1 Based on data from Na gata I., Asano H., Fujiwara K., Ruid Phase Equilib., 1, 211-217, 1977-1978
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 25% (4 reviews)

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:
Students also viewed these Engineering questions
-
In Exercises 3033, determine whether the values in each table belong to an exponential function, a logarithmic function, a linear function, or a quadratic function. r alen 3 1 3 9 27 -1 0 1 2 3
-
On February 27, 2020 Gringotts Bank gave a $1,000 loan to Hermione and took a security interest in her book collection with an agreement listing the books as collateral. Gringotts Bank agreed and...
-
Figure P2.17 shows several hypothetical velocitytime graphs. For each case, sketch qualitatively the corresponding accelerationtime graph. Figure P2.17 Case 2 Case 1 Case 3
-
print_list Favorite Language/Type: Author: Write a function named print_list that accepts a list of integers as a parameter and prints them, one per line, in the format shown. Your code should work...
-
Apple produces iPods for sale. Identify some of the variable and fixed product costs associated with that production.
-
The paper Good for Women, Good for Men, Bad for People: Simpsons Paradox and the Importance of Sex Specific Analysis in Observational Studies (Journal of Womens Health and Gender Based Medicine [...
-
Explain why the quality of tourism services is harder to define and manage than the quality of hard goods.
-
Review the Diversity Competency feature entitled Chubbs Business Case for Diversity. In what three ways do you agree and/or disagree with this business case for diversity?
-
Taveras Corporation is currently operating at 50% of its available manufacturing capacity. It uses a job-order costing system with a plantwide predetermined overhead rate based on machine-hours. At...
-
3. Figure out the adjustments and do the adjusting entries. Only Service revenue has two entries in the adjustment. HUNTER ENVIRONMENTAL CONSULTING Accounting Worksheet For the Month Ended May 31,...
-
This problem concerns the gas studied in problem 6-18, which is known to follow the EOS: V = RT/P + aP 2 where a = 0.01 L/bar2mol. A. Find a general equation for the fugacity of this compound as a...
-
In a mixing unit at your plant, you mix 40 moles/min of an equimolar mixture of benzene (1) + 2-propanol (2) at 298.15 K with 80 moles/min of pure benzene at 298.15 K. If you desire to keep the...
-
What is the projection of r(t) = ti + 14j + e1k onto the xz-plane?
-
The 60 deg strain gauge rosette is mounted on the bracket. The following readings are obtained for each gauge a = 100 106, b = 250 106, and c = 150 106. Determine: (a) the strains x, y, and xy for...
-
Assume the ledge has the dimensions shown and is attached to the building with a series of equally spaved pins around the circumference of the building. Design the pins so that the ledge can support...
-
Describe in detail about the arterial supply and venous drainage of heart with its Applied Anatomy?
-
1. Gluteus maximus muscle and mention the structures covered by it.
-
2. External features and relations of Kidney.
-
What are G words used for in NC?
-
What can you do to reduce hunger where you live? To reduce hunger globally?
-
A tall cylindrical silo carrying flour is to be supported by a 1.5 m wide ring beam that can be designed as a continuous foundation. The inner and outer diameters of the ring are 10 m and 13 m,...
-
A 2.0 m 2.0 m square pad footing will be placed in a normally consolidated clay soil to carry a column load Q. The depth of the footing is 1.0 m. The soil parameters are: c' = 0, ' = 26, = 19 kN/m...
-
An eccentrically loaded continuous foundation is shown in Figure P6.18. Determine the ultimate load Q u per unit length that the foundation can carry. Use the reduction factor method [Eq. (6.67)]....
-
Under what circumstances can a financial statement user KNOW that a company is going forward if there is a DECREASE in year-over-year retained earnings
-
Following is a series of independent cases . In each situation, indicate the cash distribution to be made to partners at the end of the liquidation process. Unless otherwise stated, assume that all...
-
ASSETS LIABILITES AND EQUITY TII TTI TIT TTT EXPENSES REVENUES Inceresumman T T

Study smarter with the SolutionInn App


