One mole of a gas is placed in a closed system with a 20 L vessel initially
Question:
One mole of a gas is placed in a closed system with a 20 L vessel initially at T = 300 K. The vessel is then isothermally expanded to 40 L. The gas follows the equation of state:
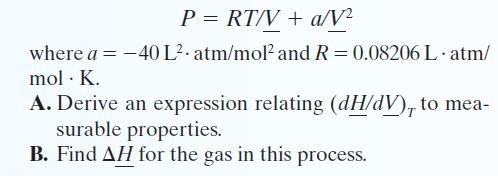
Transcribed Image Text:
P = RT/V + a/V² where a = -40 L² atm/mol² and R = 0.08206 L.atm/ mol. K. A. Derive an expression relating (dH/dV), to mea- surable properties. B. Find AH for the gas in this process.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (6 reviews)
A Deriving an expression relating dHdV to measurable properties The enthalpy change dH for an isothe...View the full answer

Answered By

Branice Buyengo Ajevi
I have been teaching for the last 5 years which has strengthened my interaction with students of different level.
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:
Students also viewed these Engineering questions
-
Euler's original article about the Konigsberg Bridge Problem, which is dated 1736, presents a second similar problem with two islands, four rivers flowing around them, and 15 bridges connecting...
-
One mole of a certain gas is contained in a vessel of volume V = 0.250 1. At a temperature T1 = 300 K the gas pressure is Pl = 90 atm, and at a temperature T2 = 350 K the pressure is p2 = 110 atm....
-
One mole of a certain ideal gas is contained under a weight-less piston of a vertical cylinder at a temperature T. The space over the piston opens into the atmosphere. What work has to be performed...
-
A house girl added 20g of sodium chloride (NaCl) to 80g of water (atomic masses are Na=23amu, Cl=35.5amu). Calculate a)Percent(w/w) of NaCl b)Mole fraction of NaCl
-
What role does the attest function play in international financial statement analysis?
-
Arakawa Pharmaceuticals of Japan is promoting a new arthritis relief drug, citing results of a recent test. The test was conducted using five patients in a test group and five patients in a control...
-
suggest how sport managers might address these trends and challenges;
-
Harrison Printing has projected its sales for the first eight months of 2014 as follows: Harrison collects 20 percent of its sales in the month of the sale, 50 percent in the month following the...
-
Use the provided information in this Excel file to find the correct answers in the indicated cells (highlighted yellow). Some information may need to be brought into this sheet from another of the...
-
A gas has an ideal gas heat capacity of C P * = (7/2)R and is described by the equation of state: Z = 1 + (CP 2 )/(RT) with C = 100 cm 3 /bar mol. A . Find a general expression for the residual...
-
Demonstrate that if a gas follows the ideal gas law, (@U/P), and (@U/V), are equal to 0.
-
What is meant by integrated system of accounting?
-
Explain product analysis
-
The accountant at EZ Toys, Inc. is analyzing the production and cost data for its Trucks Division. For October, the actual results and the master budget data are presented below. Actual Results:...
-
2. 2D Design (4 points): The Pawnee Department of Parks and Recreation has received alarming reports that their picnic tables might be unstable. Examine the picnic table design below (which weighs 50...
-
Answer 3-10 Cash flow Bailey Corporations income statement (dollars are in thousands) is given here: Sales Operating costs excluding depreciation $14,000,000 and amortization EBITDA Depreciation and...
-
You want to create a database for computer lab management. You want to keep track of the following information (Type your answer): The information about computer/workstation such as station ID,...
-
(a) What is the pressure difference required to make blood flow through an artery of inner radius 2.0 mm and length 0.20 m at a speed of 6.0 cm/s? (b) What is the pressure difference required to make...
-
Use the information given about the angles and to find the exact value of: (a) sin( + ) (b) cos( + ) (c) sin( - ) (d) tan ( + ) (e) sin(2) (f) cos (2) (g) sin /2 (h) cos/2 cos = 4/5, 0 < < /2; cos =...
-
Van der Pols equation has been used to describe many oscillatory processes. It is Plot y(t) for 3 = 1 and 0 t 20, using the initial conditions y(0) = 5, y(0) = 0. (1 ?) + 3 0 y
-
The equation of motion for a pendulum whose base is accelerating horizontally with an acceleration a(t) is Suppose that g = 9.81 m/s 2 , L " 1 m, and (0) = 0. Plot ,(t) for 0 t 10 s for the...
-
Van der Pols equation is This equation is stiff for large values of the parameter 3. Compare the performance of ode45 and ode15s for this equation. Use 3 =1000 and 0 t 3000, with the initial...
-
Question 3 A . Bonika Sdn Bhd ( BSB ) manufactures small camping tents. BSB produced 3 , 0 0 0 units during the year. These camping tents sell for RM 1 5 0 each. BSB had 5 0 0 units in finished goods...
-
Required information (The following information applies to the questions displayed below.) Davis Stores sells clothing in 15 stores located around the southwestern United States. The managers at...
-
In activity-based costing, the cost driver rate is computed by dividing the total cost per activity by the estimated number of units produced. True or False

Study smarter with the SolutionInn App


