A gas has an ideal gas heat capacity of C P * = (7/2)R and is described
Question:
A gas has an ideal gas heat capacity of CP* = (7/2)R and is described by the equation of state: Z = 1 + (CP 2)/(RT) with C = 100 cm3/bar · mol.
A. Find a general expression for the residual molar enthalpy for this gas.
B. Find a general expression for the residual molar entropy for this gas.
C. Find ΔH and ΔS for the gas if it is isothermally compressed from P = 1 bar and T = 400 K to P = 50 bar and T = 400 K. Problems 14 through 17 involve a gas that follows the equation of state: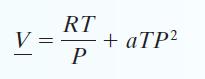 with a = 0.3 cm3/mol · bar 2 · K. The gas has a molecular mass of 120 g/mol and an ideal gas heat capacity CP* = 40 J/mol.
with a = 0.3 cm3/mol · bar 2 · K. The gas has a molecular mass of 120 g/mol and an ideal gas heat capacity CP* = 40 J/mol.
Transcribed Image Text:
V RT P + aTP²
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
Lets start by finding the general expressions for the residual molar enthalpy H and the residual molar entropy S for the given gas using the provided ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:
Students also viewed these Engineering questions
-
1 kg of nitrogen is contained in a piston-cylinder device. The nitrogen is isothermally compressed from P = 1 bar to P = 10 bar at T = 300 K. Find the initial volume, final volume, and work and heat...
-
A certain nonideal gas is described by the equation of state where T is the temperature on the Kelvin scale, V m is the molar volume, P is the pressure, and R is the gas constant. For this gas, the...
-
?
-
What is internal control, how do internal auditors relate to it, and how does this process relate to the analysis of financial statements?
-
Goveia Inc. is evaluating three possible bonus incentive programs for its sales staff. During a trial period lasting four months, five sales staff members were randomly assigned to each bonus...
-
highlight opportunities for the development of managerial practice and academic research as a result of this book.
-
Shalimar Company manufactures and sells industrial products. For next year, Shalimar has budgeted the follow sales: Quarter 1 ......$4,600,000 Quarter 2 ...... 5,100,000 Quarter 3 ...... 5,000,000...
-
Calculate the current ratio and the quick ratio for the following partial financial statement for Tootsie Roll. (Round your answers to the nearest hundredth.) Assets Liabilities Current assets:...
-
Methane enters a process at T = 300F and P = 1 atm, and is heated and compressed to T = 400F and P = 5 atm. Find the change in molar Gibbs free energy for the methane, using Figure 7-1. FIGURE 7-1...
-
One mole of a gas is placed in a closed system with a 20 L vessel initially at T = 300 K. The vessel is then isothermally expanded to 40 L. The gas follows the equation of state: P = RT/V + a/V where...
-
Linebarger Corporation has net income of $11.44 million and net revenue of $95 million in 2017. Its assets are $14 million at the beginning of the year and $18 million at the end of the year. What...
-
i) Generate a third degree polynomial in x and y named g(x, y) that is based on your mobile number (Note: In case there is a 0 in one of the digits replace it by 3). Suppose your mobile number is...
-
A pistoncylinder device contains 0.85 kg of refrigerant-134a at 210 oC. The piston that is free to move has a mass of 12 kg and a diameter of 25 cm. The local atmospheric pressure is 88 kPa. Now,...
-
3.3. Using the BEMT, show the effect of increasing linear twist on the variations in inflow, thrust, induced power, profile power, and lift coefficient across the span of a rotor with four blades of...
-
By uploading this work, I attest that the work contained herein is solely my own, that I only used the given equation sheet as a reference, and that I have not received any information from anyone...
-
Demand for patient surgery at Washington General Hospital has increased steadily in the past few years, as seen in the following table: ...
-
A lid is put on a box that is 15 cm long, 13 cm wide, and 8.0 cm tall and the box is then evacuated until its inner pressure is 0.80 105 Pa. How much force is required to lift the lid (a) At sea...
-
Establish identity. cos( + k) = (-1)k cos , k any integer
-
The following equation describes the motion of a certain mass connected to a spring, with viscous friction on the surface where f(t) is an applied force. Suppose that f (t) = 0 for t + 0 and f (t) =...
-
The following equation describes the motion of a certain mass connected to a spring, with viscous friction on the surface where f(t) is an applied force. Suppose that f(t) = 0 for t + 0 and f(t) = 10...
-
The following equation describes the motion of a certain mass connected to a spring, with no friction where f(t) is an applied force. Suppose the applied force is sinusoidal with a frequency of ...
-
Upon graduating from High School in 2019, you were granted a $6,000 college scholarship for academic year 2019-2020. As a full-time student and degree candidate during the fall term of 2019 you...
-
REED COMPANY December 31 2021 2020 Sales revenue $4,400,000 $3,500,000 Cost of goods sold 2,860,000 2,000,000 Administrative expense 800,000 675,000 Selling expense 360,000 302,000 Interest revenue...
-
Jimmy earned $85,000 in salary from his job as an engineer. He earned $3,000 in interest income. He made RPP contributions of $2,119 and contributed $15,000 to his RRSP. Given the following schedule...

Study smarter with the SolutionInn App


