Methane enters a process at T = 300F and P = 1 atm, and is heated and
Question:
Methane enters a process at T = 300°F and P = 1 atm, and is heated and compressed to T = 400°F and P = 5 atm. Find the change in molar Gibbs free energy for the methane, using Figure 7-1.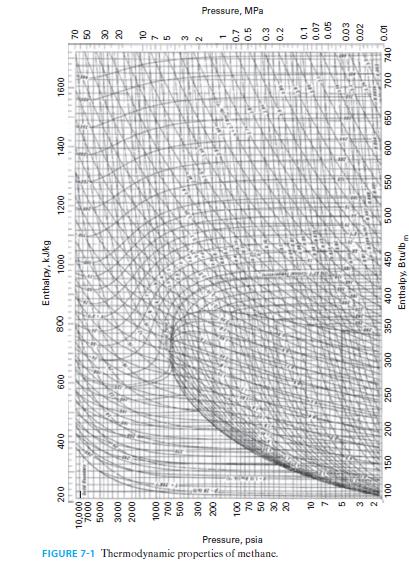
Transcribed Image Text:
FIGURE 7-1 Thermodynamic properties of methane. Pressure, psia 200 10,000 7000 17 5000 3000 2000 1000 27 5 32 One 100 150 400 MALA 600 200 250 300 800 350 Enthalpy, kJ/kg 1000 400 450 Enthalpy, Btu/lb, 1200 1+12 Lehek 500 550 1400 1600 1802 650 600 SEENE ४ ४ ४ ४ 215 NW 700 740 0.01 Pressure, MPa
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
To find the change in molar Gibbs free energy for the methane we can use the following equation G H ...View the full answer

Answered By

Danish Sohail
My objective is to become most reliable expert for clients. For last 10 years I have been associated with the field of accounting and finance. My aim is to strive for best results and pay particular attention to client needs. I am always enthusiastic to help clients for issues and concerns related to business studies. I can work on analysis of the financial statements, calculate different ratios and analysis of ratios. I can critically evaluate stock prices based on the financial analysis and valuation for companies using financial statements of the business entity being valued with use of excel tools. I have expertise to provide effective and reliable help for projects in corporate finance, equity investments, financial accounting, cost accounting, financial planning, business plans, marketing plans, performance measurement, budgeting, economic research, risk assessment, risk management, derivatives, fixed income investments, taxation, auditing, and financial performance analysis.
4.80+
78+ Reviews
112+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:
Students also viewed these Engineering questions
-
Determine the change in molar Gibbs Free Energy of liquid water at 25C as pressure increased from 1 bar to 10 bar. Repeat for steam assuming ideal gas under same condition.
-
That problem asked you to estimate the change in molar enthalpy when hydrogen is compressed from T = 300 K and P = 1 bar to T = 700 K and P = 200 bar. At that time, the ideal gas model was the only...
-
You are designing a process in which benzene is used as a solvent. In order to size process equipment, you need to know the properties at several conditions. Estimate the following quantites. A. The...
-
96. A 66-year-old woman with a long history of heavy smoking presents to her doctor with complaints of shortness of breath and chronic coughing that has been present for about 2 years and has been...
-
Condensed comparative income statements of Senorina Panchos, a Mexican restaurant chain, for the years 2009 through 2011 are presented in Exhibit 9-18 (000,000s pesos). You are interested in gauging...
-
You have just completed a regression study in which you are attempting to link weekly sales of SeaFarers new sun block spray to three factors: price, advertising and use of a special point of...
-
What do you think this case tells us about the future of motorsport and of sport in general?
-
Stine Manufacturing uses a job order cost system. On May 1, the company has a balance in Work in Process Inventory of $3,980 and two jobs in process: Job No. 429 $2,590 and Job No. 430 $1,390. During...
-
Problem 10-33 Direct-Material and Direct-Labor Variances (LO 10-1, 10-3) New Jersey Valve Company manufactured 7,800 units during January of a control valve used by milk processors in its Camden...
-
A gas with a flow rate of 300 mol/min enters a steady-state, adiabatic nozzle with negligible velocity at T = 500 K and P = 10 bar and leaves the nozzle at P = 1 bar. The gas has C P * = 40 J/mol K....
-
A gas has an ideal gas heat capacity of C P * = (7/2)R and is described by the equation of state: Z = 1 + (CP 2 )/(RT) with C = 100 cm 3 /bar mol. A . Find a general expression for the residual...
-
A passenger on an airplane flying across the Atlantic receives an extra radiation dose of about \(5 \mu \mathrm{Sv}\) per hour from cosmic rays. How many hours of flying would it take in one year for...
-
Part A At a given instant A has the motion shown in (Figure 1). Determine the acceleration of B at this instant. Express your answer in feet per second squared to three significant figures. Enter...
-
The balances of selected accounts of Casper Company on February 28, 20X1, were as follows: Sales $250,000 and Sales Returns and Allowances $4,000. The firm's net sales are subject to an 7 percent...
-
1. Draw and label force diagrams for the physics book and for the calculator. Add equality marks showing any equalities between force diagrams. Circle and label any Newton's third law pairs. (6 pts)...
-
Consider the Lincoln Tunnel, which was built in 1939 under the Hudson River in New York. Assume the tunnel to be empty with perfectly conducting walls and rectangular cross section with width 6.55 m...
-
Examine a well-known principal-agent contract, the sale of your home by a licensed realtor. You will use the following data to analyze this case. Your home is the typical home, approximately 1,875 sq...
-
(a) Since the flow rate is proportional to the pressure difference, show that Poiseuille's law can be written in the form P = IR, where I is the volume flow rate and R is a constant of...
-
The figure shows six containers, each of which is filled from the top. Assume that water is poured into the containers at a constant rate and each container is filled in 10 seconds. Assume also that...
-
The equation for the voltage y across the capacitor of an RC circuit is where !(t) is the applied voltage. Suppose that RC = 0.2 s and that the capacitor voltage is initially 2 V. Suppose also that...
-
The equation describing the water height h in a spherical tank with a drain at the bottom is Suppose the tanks radius is r = 3 m and the circular drain hole has a radius of 2 cm. Assume that C d =...
-
The following equation describes a certain dilution process, where y(t) is the concentration of salt in a tank of freshwater to which salt brine is being added. Suppose that y(0) = 0. Plot y(t) for 0...
-
Arlo Tech Inc. has an Automotive and a Consumer Products Division. The two divisions share a distribution warehouse in another city. The space in the warehouse is allocated 60% to Automotive and 40%...
-
At the beginning of September, Helen Rojas started Rojas Wealth Management Consulting, a firm that offers financial planning and advice about investing and managing money. On September 30, the...
-
Empire Polymer Development produces three products using three different continuous processes. The products are Yarex, Darol, and Norex. Projected sales in gallons for the three products for the...

Study smarter with the SolutionInn App


