The classic way to synthesize ammonia is through the gas phase chemical reaction: N, + 3H,2NH, This
Question:
The classic way to synthesize ammonia is through the gas phase chemical reaction: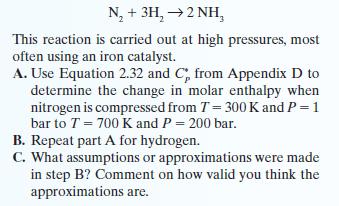
Transcribed Image Text:
N, + 3H,→2NH, This reaction is carried out at high pressures, most often using an iron catalyst. A. Use Equation 2.32 and C, from Appendix D to determine the change in molar enthalpy when nitrogen is compressed from T = 300 K and P = 1 bar to T = 700 K and P = 200 bar. B. Repeat part A for hydrogen. C. What assumptions or approximations were made in step B? Comment on how valid you think the approximations are.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To calculate the change in molar enthalpy AH using Equation 232 which is the definition of molar ent...View the full answer

Answered By

Daniel Kimutai
I am a competent academic expert who delivers excellent writing content from various subjects that pertain to academics. It includes Electronics engineering, History, Economics, Government, Management, IT, Religion, English, Psychology, Sociology, among others. By using Grammarly and Turnitin tools, I make sure that the writing content is original and delivered in time. For seven years, I have worked as a freelance writer, and many scholars have achieved their career dreams through my assistance.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:
Students also viewed these Engineering questions
-
The synthesis of ammonia gas from nitrogen gas and hy-drogen gas represents a classic case in which a knowl-edge of kinetics and equilibrium was used to make a desired chemical reaction economically...
-
Various uses for nitric acid are given in Problem 6.43, along with information about how this important chemical is synthesized industrially. The key reactions are oxidations of ammonia to nitric...
-
Nitric acid is a chemical intermediate primarily used in the synthesis of ammonium nitrate, which is used in the manufacture of fertilizers. The acid also is important in the production of other...
-
Because the entries in the present value table (Table 13 - 3) are reciprocals of the corresponding entries in the future value table (Table 13 - 1), how can Table 13 - 3 be used to find the future...
-
What role do the United Nations and the Organization for Economic Cooperation and Development play in harmonizing accounting and auditing standards?
-
As discussed in the chapter, companies are often classified into one of three categories: service, merchandising, and manufacturing. Required: 1. Choose one well-known company from each category and...
-
Visit the NBAs Basketball Without Borders website (www.nba.com/bwb/). Evaluate what publics the NBA is trying to reach with this community relations activity. How would you enhance the leagues...
-
The Canton Corporation shows the following income statement. The firm uses FIFO inventory accounting. CANTON CORPORATION Income Statement for 2013 Sales $272,800 (17,600 units at $15.50) Cost of...
-
Anheuser-Busch is considering introducing a new ginger beer line for the holidays and provides the following data about this potential project. If the total project life is 20 years and the company...
-
The specific enthalpy of liquid water at typical ambient conditions, like T = 25C and P = 1 bar, is not given in the steam tables. However, the specific enthalpy of saturated liquid at P = 1 bar is...
-
A refrigeration process includes a compressor, as explained in detail in Chapter 5, because it is necessary to change the boiling point of the refrigerant, which is done by controlling the pressure....
-
In 20X2, Clarkson Inc. initiated a full-scale, quality improvement program. At the end of the year, Tony Ming, the president, noted with some satisfaction that the defects per unit of product had...
-
Alvarado Company produced 6,400 units of product that required 5.5 standard direct labor hours per unit. The standard variable overhead cost per unit is $5.80 per direct labor hour. The actual...
-
A company must decide between scrapping or reworking units that do not pass inspection. The company has 16,000 defective units that have already cost $132,000 to manufacture. The units can be sold as...
-
according to the phase rule, the triple point of a pure substance is A. invariant B. u nivariant C. bivariant D. none of the above
-
33. If the equipment in the previous question had sold for $15,000, the correct entry would be: a. Cash debit $15,000. Gain credit $3,000. $12,000 Equipment credit b. Cash debit $15,000. Debit a loss...
-
The banks play a central role in financial intermediation in New Zealand. 1.What is financial intermediation? Who performs it? and why is it important? 2.What is Qualitative Asset transformation...
-
A garden hose of inner radius 1.0 cm carries water at 2.0 m/s. The nozzle at the end has radius 0.20 cm. How fast does the water move through the nozzle?
-
Write the binomial probability in words. Then, use a continuity correction to convert the binomial probability to a normal distribution probability. P(x 110)
-
Show that R -1 (a) = R(-a). This equation shows that a rotation through a negative angle is equivalent to an inverse transformation.
-
Find the characteristic polynomial and roots of the following matrix: -- A = 21 3k -7 .
-
Use the matrix inverse and the matrix division method to solve the following set for x and y in terms of c: 4cx + 5y = 43 3x 4y = -22
-
What provides the user with the highest level of assurance that the financial statements are free from material misstatement and/or that the financial statements do not require a material...
-
Are the following required to file a Federal return? Answer ( YES or NO ) HOH, age 29, gross income $18,850. Choose one answer. a. Yes b. No
-
Consider how Cherry Valley, a popular ski resort, could use capital budgeting to decide whether the $ 9 million Waterfall Park Lodge expansion would be a good investment. ( Click the icon to view the...

Study smarter with the SolutionInn App


