The surface of a catalytic slab is exposed to a concentration distribution (c_{a o}(x)). As material diffuses
Question:
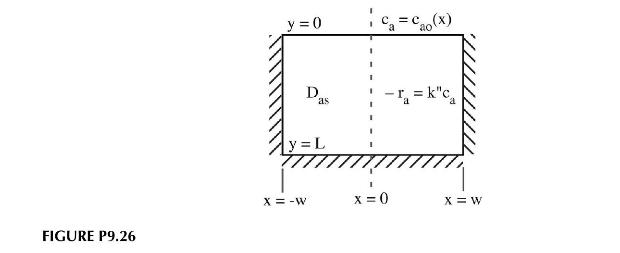
The surface of a catalytic slab is exposed to a concentration distribution \(c_{a o}(x)\). As material diffuses into the slab it reacts according to a first-order chemical reaction \((a \rightarrow b)\). The sides and bottom of the catalyst are impermeable and \(a\) exists in dilute solution in the slab as shown in Figure P9.26.
a. Derive the partial differential equation governing the concentration profile inside the slab.
b. Solve the differential equation for the concentration field.
c. What is the flux of material through the exposed surface?
d. How much unreacted material is inside the slab?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





