This problem re-examines the dirigible in Example 2-4. The balloon contains 5000 moles of helium, which can
Question:
This problem re-examines the dirigible in Example 2-4. The balloon contains 5000 moles of helium, which can be modeled as an ideal gas with CP* = (5/2)R at the conditions in this problem. The dirigible is initially at T = 25°C and P = 1 atm. The dirigible flies slowly enough that all changes to the helium are reversible. Find Q, W, ΔU,ΔH, and ΔS for the helium if its final state is:
A. T = 15°C and P = 1 atm. Pressure is uniform throughout the process.
B. T = 25°C and P = 0.9 atm. Temperature is uniform throughout the process.
Example 2-4
The balloon portion of a dirigible is filled with 5000 moles of helium (Figure 2-13). Ini tially, the helium is at P = 1 atm and T = 25°C. As the dirigible gains altitude, the pres sure drops to P = 0.95 atm, and the temperature drops to T = 15°C. Find the changes in volume, internal energy, and enthalpy for the helium in this process, assuming helium has a constant ideal gas heat capacity of C*V = (1.5)R. 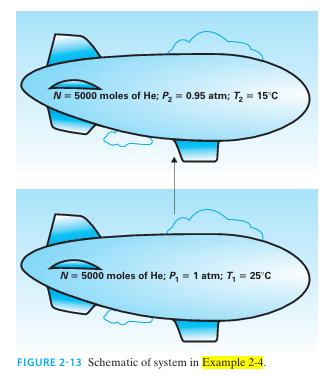
Step by Step Answer:

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco





