Two moles of an ideal gas are confined in a piston-cylinder device, initially at P = 5
Question:
Two moles of an ideal gas are confined in a piston-cylinder device, initially at P = 5 bar and T = 300 K. The surroundings are also at T = 300 K. The atmosphere is at P=1 bar but clamps hold the piston in place, as shown in Figure 4-2. When the clamps are removed, the gas expands rapidly—so rapidly that heat transfer is negligible during this step—until the pressure of the gas is P = 1 bar. Heat is then transferred to the gas from the surroundings until the gas reaches a final state of P = 1 bar and T = 300 K. Find the total work done by the gas on the surroundings, and the total heat transferred to the gas.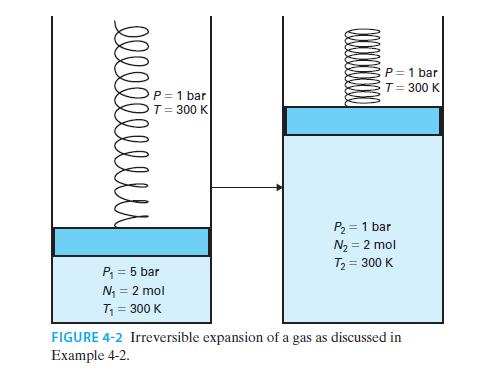
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:





