Vessel A contains 100 g of water initially at 80C and Vessel B contains 50 g of
Question:
“Vessel A” contains 100 g of water initially at 80°C and “Vessel B” contains 50 g of water initially at 20°C. They are brought into contact as shown in Figure 4-6, and allowed to reach equilibrium. Find the final temperature of the water and the change in entropy of the universe. Assume the heat capacity and specific volume of water are constant with respect to temperature (use CP ~ CV ~ 4.2 J/g · K), and that no heat exchange with the surroundings occurs.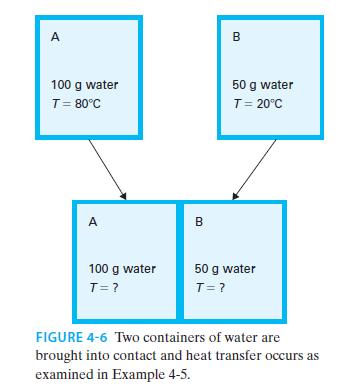
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:





