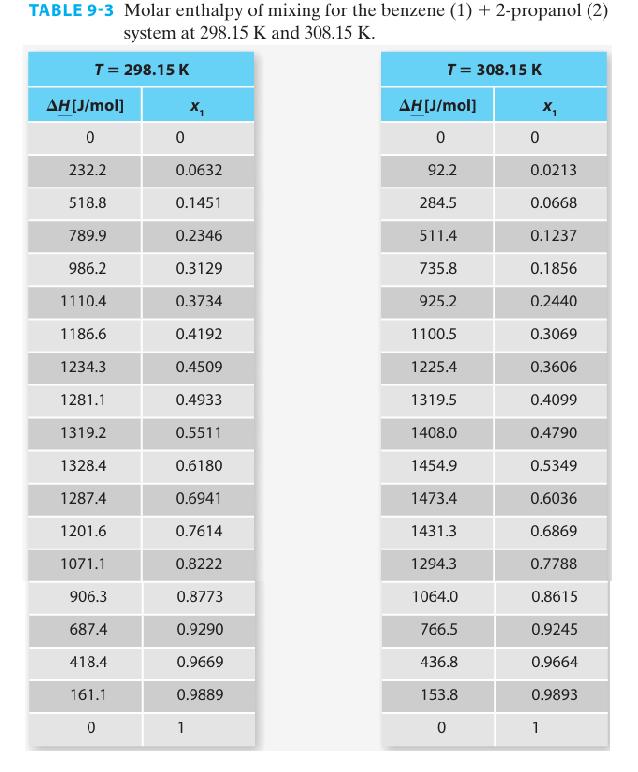
Using the data for the benzene (1) + 2-propanol (2) system in Table 9-3, fit the experimental
Question:
Using the data for the benzene (1) + 2-propanol (2) system in Table 9-3, fit the experimental data to an appropriate polynomial equation whose independent variable is the mole fraction of ethanol. Work with only the 298.15 K data set.
Transcribed Image Text:
TABLE 9-3 Molar enthalpy of mixing for the benzene (1) + 2-propanol (2) system at 298.15 K and 308.15 K. T = 298.15 K ΔΗ[J/mol] 0 232.2 518.8 789.9 986.2 1110.4 1186.6 1234.3 1281.1 1319.2 1328.4 1287.4 1201.6 1071.1 906.3 687.4 418.4 161.1 0 0 X₁ 0.0632 0.1451 0.2346 0.3129 0.3734 0.4192 0.4509 0.4933 0.5511 0.6180 0.6941 0.7614 0.8222 0.8773 0.9290 0.9669 0.9889 1 T = 308.15 K AH[J/mol] 0 92.2 284.5 511.4 735.8 925.2 1100.5 1225.4 1319.5 1408.0 1454.9 1473.4 1431.3 1294.3 1064.0 766.5 436.8 153.8 0 0 X₁ 0.0213 0.0668 0.1237 0.1856 0.2440 0.3069 0.3606 0.4099 0.4790 0.5349 0.6036 0.6869 0.7788 0.8615 0.9245 0.9664 0.9893 1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)

Answered By

Kainat Shabbir
i am an experienced qualified expert with a long record of success helping clients overcome specific difficulties in information technology, business and arts greatly increasing their confidence in these topics. i am providing professional services in following concerns research papers, term papers, dissertation writing, book reports, biography writing, proofreading, editing, article critique, book review, coursework, c++, java, bootstarp, database.
5.00+
184+ Reviews
255+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:
Students also viewed these Engineering questions
-
Using the information from Table 9-3, plot the partial molar enthalpies of both components at both temperatures on the same curve as a function of composition. You may have already completed some of...
-
In the process of distillation, a mixture of two (or more) volatile liquids is first heated to convert the volatile materials to the vapor state. Then the vapor is condensed, reforming the liquid....
-
Using the data for t he coffee table in problem 14.26, build a labor schedule when the labor standard for each top is 2 labor hours; each leg including brass cap installation requires ¼ hours,...
-
Find any horizontal or vertical asymptotes. f(x) = = X 1- X
-
Why do people differ in the types of needs they are trying to satisfy at work?
-
In exercise 5, the owner of Showtime Movie Theaters, Inc., used multiple regression analysis to predict weekly gross revenue (y) as a function of television advertising (x1) and newspaper advertising...
-
If a firm borrowed $500,000 at a rate of 10% simple interest with monthly interest payments and a 365-day year, what would be the required interest payment for a 30-day month? If interest must be...
-
Daniel Singh, the controller of Meier Corporation, is trying to prepare a sales budget for the coming year. The income statements for the last four quarters follow. Historically, cost of goods sold...
-
Record the journal entries for January sales and credit card collections. The company had sales on account (customer and credit cards) of $142,298. Record the sales on account. The company does not...
-
What is the entropy change when you mix 1 mole of 1-propanol with 4 moles of ethanol at 25C and 1 atm? Assume an ideal solution.
-
You are designing a fermentation process that produces mixtures of water, acetone, ethanol, and 1-butanol. You are considering whether distillation will be an effective means of separating these...
-
You developed an interactive GUI application for Carlys Catering. Now, design a JPanel that uses graphics to display a logo for the company, and modify the GUI application to include it. Save the...
-
What is a view? What is a viewpoint? What is a visualization? How do they come together to help communicate a model to the stakeholders?
-
A spray dryer receives distillers dried grains (DDGS) with a 65% moisture content. The spray dryer operates with dry air entering at 180 C, 1 bar, and moist air exits at 87 C, 1 bar, and 25% relative...
-
Lifestyle is how one enacts the self-concept. The way they would enact it is through buying luxury items which is the most premium iPhone. The latent reasons why people want an iPhone 15 all have to...
-
Make a Tows Matrix that assess the strengths, weakness, opportunities, and threats for Dannon based on the case study For typical corporate strategies under purpose of communication. Strengths 1) 2)...
-
Now that you've watched the lectures, The Abilene Paradox movie, and the Challenger Disaster Video, I'd like you to think for a moment about when you may have observed the Abilene Paradox or...
-
Which compound is more likely to be carcinogenic? b. or
-
How can NAFTA be beneficial to suppliers of Walmart?
-
Difference plot. A solution containing 3.96 mmol acetic acid plus 0.484 mmol HCI in 200 mL, of 0.10 M KC1 was titrated with 0.490 5 M NaOH to measure K. for acetic acid. (a) Write expressions for the...
-
Difference plot. A solution containing 3.96 mmol acetic acid plus 0.484 mmol HCI in 200 mL, of 0.10 M KC1 was titrated with 0.490 5 M NaOH to measure K. for acetic acid. (a) Write expressions for the...
-
Why does the solubility of a salt of a basic anion increase with decreasing pH? Write chemical reactions for the minerals galena (PbS) and cerussite (PbCO 3 ) to explain how acid rain mobilizes...
-
Complete the following sentences by anyone of the following terms. Each term can be used more than one time or not used at all. Group Code Data Processing Cycle Data Value Transaction File General...
-
5 What's the biggest source of credit card fraud? List at least five things you can do to reduce your chances of being a victim of credit card fraud. 6 Distinguish between a Wage Earner Plan and...
-
Bob Night opened The General's Favorite Fishing Hole. The fishing camp is open from April through September and attracts many famous college basketball coaches during the off- season. Guests...

Study smarter with the SolutionInn App


