Using the NIST Webbook, if one looks up the molar enthalpy of pure benzene at 308.15 K
Question:
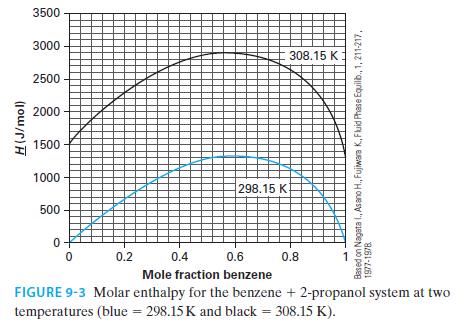
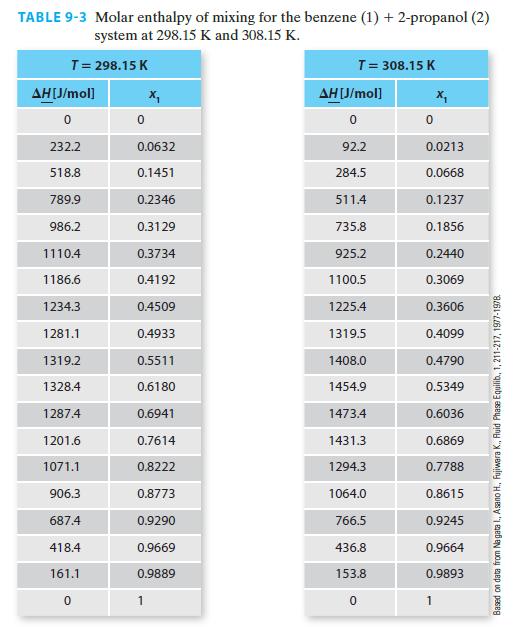
Using the NIST Webbook, if one looks up the molar enthalpy of pure benzene at 308.15 K and 1 bar, the reported value is –6359.6 J/mol. Figure 9-3 has this same property as being equal to 1356.9 J/mol (via Example 9-2). Whose value, if any, is in error? Please explain your answer.
Transcribed Image Text:
H(J/mol) 3500 3000 2500 2000 1500 1000 500 0 0 298.15 K 0.2 308.15 K 0.4 0.6 Mole fraction benzene FIGURE 9-3 Molar enthalpy for the benzene + 2-propanol system at two temperatures (blue = 298.15 K and black = 308.15 K). X 0.8 Based on Nagata I., Asano H., Fujiwara K., Fluid Phase Equilib., 1, 211-217, 1977-1978 1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:
Students also viewed these Engineering questions
-
Suppose that the depreciation of EA/B was 7% over the past year, and prices in country A increased by 4% from this time last year. Assuming relative PPP holds, what was the rate of inflation in...
-
Marili had a full life with her husband, Jack and their 3 adult daughters (Jackie, Sophie, and Stephanie). Jackie has 2 children and Stephanie had 2 children. Unfortunately, Stephanie passed away 2...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Why does Erasmus attack Church officials in his In Praise of Folly? O For spending money on lavish art O For not allowing clergy to marry O For supporting military campaigns O For selling pardons and...
-
Your company has just decided to purchase 50 percent of its inventory from China and purchases will be invoiced in Chinese yuan. What four processes do you need to consider in designing a foreign...
-
The manufacturer of Boston and Vermont asphalt shingles provides its customers with a 20- year warranty on most of its products. To determine whether a shingle will last through the warranty period,...
-
18.9 What is the direct labour rate variance for the month? a) 901 (A) b) 795 (A) c) 795 (F) d) 901 (F).
-
Lakeway Manufacturing Co. manufactures and sells household cleaning products. The company's research department has developed a new cleaner for which a standard cost must be determined. The new...
-
The growth in assurance-type work provides great money-spinning opportunity for audit firms to provide a lower level of assurance, involving less work and reduced engagements risks, compared to the...
-
You have 100 grams of water at 25C in a container that holds exactly 200 ml. What mass of methanol do you need to add to the system such that the container is filled without overflowing? See the...
-
This problem involves the same compound that was examined in Problems 6-14 through 6-17, which in the vapor phase was described by the EOS: A. The fugacity in the vapor phase at T = 50C and P = 0.1...
-
Suppose that a random variable X has the Bernoulli distribution with parameter p = 0.7. Sketch the c.d.f. of X.
-
5 28 its Jay Oullette, CEO of Bumper to Bumper Incorporated, anticipates that his company's year-end balance sheet will show current assets of $12,801 and current liabilities of $7,540. Oullette has...
-
Test the given claim. Assume that a simple random sample is selected from a normally distributed population. Use either the P-value method or the traditional method of testing hypotheses. Company A...
-
Trojan Technologies As Joyce Guo, senior buyer at Trojan Technologies Inc. in London, Ontario, Canada, finished her presentation, Randy Haill, materials manager, Made the following comments to her:...
-
In 2022, Andrew, who is single, has a comfortable salary from his job as well as income from his investment portfolio. However, he is habitually late in filing his federal income tax return. He did...
-
Express the confidence interval (0.045,0.123) in the form of p^ - E < p < p^+ E.
-
Are evenly spaced specific gravity markings on the cylinder of a hydrometer equal distances apart? In other words, is the depth d to which the cylinder is submerged linearly related to the density r...
-
Prove the result that the R 2 associated with a restricted least squares estimator is never larger than that associated with the unrestricted least squares estimator. Conclude that imposing...
-
The useful life of a machine bearing depends on its operating temperature, as the following data show. Obtain a functional description of these data. Plot the function and the data on the same plot....
-
A certain electric circuit has a resistor and a capacitor. The capacitor is initially charged to 100 V. When the power supply is detached, the capacitor voltage decays with time, as the following...
-
The distance a spring stretches from its free length is a function of how much tension force is applied to it. The following table gives the spring length y that was produced in a particular spring...
-
(i) (Click the icon is view the (Assume bonds payable are a ) nearest whole dollar.) More info a. Issuance of the bonds on January 1,2018. b. Payment of interest and amortizaton on dune 30,2018. c....
-
The Gilmore Insurance Group earned a profit of $10,000 in its first month of business. Then, its profit increased by 3% each month for the next two years. What is the total profit that the Gilmore...
-
BluStar Company has two service departments, Administration and Accounting, and two operating departments, Domestic and International. Administration costs are allocated on the basis of employees,...

Study smarter with the SolutionInn App


