You are working for a contract research firm, DATATEC, and your first job is to determine the
Question:
You are working for a contract research firm, DATATEC, and your first job is to determine the diffusion coefficient and reaction rate constant for a decomposition reaction \((A \rightarrow C)\). You propose to do this in a diffusion cell where you introduce a small amount of A into a cell filled with solvent, B. The A diffuses through the cell and reacts along the way. At the exit of the cell, the reaction is complete. You have a spectrophotometer that lets you isolate a slice of the cell and measure how much A is remaining there. Assuming you know the concentration of \(\mathrm{A}\) at the inlet to the cell:
a. What type of model will you use to analyze the data? Formulate and solve it.
b. What assumptions must you make to use your model?
c. Can you determine the diffusion coefficient and reaction rate constant independently? Why?
d. Given the experimental data shown below, what can you tell about the diffusion coefficient and the reaction rate constant?
\[c_{a o}=1.0 \times 10^{-3} \mathrm{moles} / \mathrm{m}^{3} \quad L=0.06 \mathrm{~m} \quad A_{o}=1.0 \times 10^{-4} \mathrm{~m}^{2}\]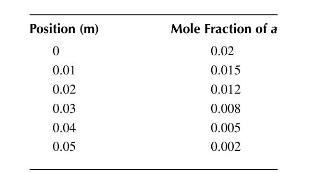
Step by Step Answer:






