An energy storage system is proposed to absorb thermal energy collected during the day with a solar
Question:
An energy storage system is proposed to absorb thermal energy collected during the day with a solar collector and release thermal energy at night to heat a building. The key component of the system is a shellandtube heat exchanger with the shell side filled with -octadecane (see Problem 8.47).
Data From Problem 8.47
Consider a horizontal, thin-walled circular tube of diameter D = 0.025 m submerged in a container of noctadecane (paraffin), which is used to store thermal energy. As hot water flows through the tube, heat is transferred to the paraffin, converting it from the solid to liquid state at the phase change temperature of T∞ = 27.4°C. The latent heat of fusion and density of paraffin are hsf = 244 kJ/kg and p = 770 kg/m3, respectively, and thermophysical properties of the water may be taken as cp = 4.185 kJ/kg · K, k = 0.653 W/m · K, μ = 467 X 10–6 kg/s · m, and Pr = 2.99.
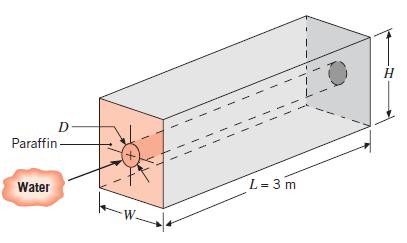
(a) Assuming the tube surface to have a uniform temperature corresponding to that of the phase change, determine the water outlet temperature and total heat transfer rate for a water flow rate of 0.1 kg/s and an inlet temperature of 60°C. If H = W = 0.25 m, how long would it take to completely liquefy the paraffin, from an initial state for which all the paraffin is solid and at 27.4°C?
(b) The liquefaction process can be accelerated by increasing the flow rate of the water. Compute and plot the heat rate and outlet temperature as a function of flow rate for 0.1 ≤ m ≤ 0.5 kg/s. How long would it take to melt the paraffin for m = 0.5 kg/s?
Step by Step Answer:

Fundamentals Of Heat And Mass Transfer
ISBN: 9780470501979
7th Edition
Authors: Theodore L. Bergman, Adrienne S. Lavine, Frank P. Incropera, David P. DeWitt





