A process is being developed to produce carbonated beverages. As part of this process, a wetted-wall absorption
Question:
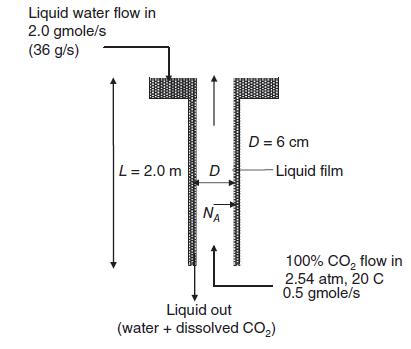
A process is being developed to produce carbonated beverages. As part of this process, a wetted-wall absorption column 2.0 m long will be used to dissolve carbon dioxide (CO2) gas into water. The process is shown in the figure (next page). In this process, pure mountain spring water containing no dissolved CO2 enters the top of the column at a flow rate of 2.0 gmole/s (36.0 g/s). Pure, 100% CO2 gas at 2.54 atm is also fed to the bottom of the column at a flow rate of 0.5 gmole/s. As the liquid flows down the wetted-wall column, CO2 gas dissolves into the water. The carbonated water exits the bottom of the column, and the unused CO2 gas exits the top of the column. The inner column diameter is 6.0 cm. The temperature is aintained at 20 C. At 20 C, the Henry’s law constant for the dissolution of CO2 gas in water is 25.4 atm m3/kgmole. The molar density of liquid water is 55.5 kgmole/m3 at 20 C, the mass density of liquid water is 998.2 kg/m3 at 20 C, the viscosity of liquid water is 993 X 10–6 kg/m s at 20 C. Water has a vapor pressure at 20 C. However, for this problem you may assume that the water solvent is essentially nonvolatile, so that 100% CO2 gas composition is always maintained down the length of the column.
a. What is the maximum possible concentration of dissolved CO2 in water at 2.54 atm CO2 partial pressure?
b. What is the liquid-phase mass-transfer coefficient for this process?
c. What is the exit concentration of dissolved CO2 in the carbonated water if the wetted-wall column is 2.0 m in length?
Step by Step Answer:

Fundamentals Of Momentum Heat And Mass Transfer
ISBN: 9781119723547
7th Edition
Authors: James Welty, Gregory L. Rorrer, David G. Foster





