A400-L tank,A(see Fig. P3.47), contains argon gas at 250 kPa and 30C. Cylinder B, having a frictionless
Question:
A400-L tank,A(see Fig. P3.47), contains argon gas at 250 kPa and 30◦C. Cylinder B, having a frictionless piston of such mass that a pressure of 150 kPa will float it, is initially empty. The valve is opened, and argon flows into B and eventually reaches a uniform state of 150 kPa and 30◦C throughout. What is the work done by the argon?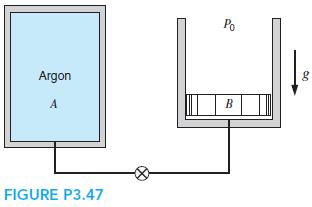
Transcribed Image Text:
Po Argon g A B FIGURE P3.47
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (14 reviews)

Answered By

Marvine Ekina
Marvine Ekina
Dedicated and experienced Academic Tutor with a proven track record for helping students to improve their academic performance. Adept at evaluating students and creating learning plans based on their strengths and weaknesses. Bringing forth a devotion to education and helping others to achieve their academic and life goals.
PERSONAL INFORMATION
Address: , ,
Nationality:
Driving License:
Hobbies: reading
SKILLS
????? Problem Solving Skills
????? Predictive Modeling
????? Customer Service Skills
????? Creative Problem Solving Skills
????? Strong Analytical Skills
????? Project Management Skills
????? Multitasking Skills
????? Leadership Skills
????? Curriculum Development
????? Excellent Communication Skills
????? SAT Prep
????? Knowledge of Educational Philosophies
????? Informal and Formal Assessments
0.00
0 Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag
Question Posted:
Students also viewed these Sciences questions
-
A 3-m3 rigid tank contains hydrogen at 250 kPa and 550 K. The gas is now cooled until its temperature drops to 350 K. Determine (a) The final pressure in the tank and (b) The amount of heat transfer.
-
A piston/cylinder setup contains argon gas at 140 kPa, 10C, and the volume is 100 L. The gas is compressed in a polytropic process to 700 kPa, 280C. Calculate the heat transfer during the process.
-
A 400-L tank, A contains argon gas at 250 kPa, 30C. Cylinder B, having a frictionless piston of such mass that a pressure of 150 kPa will float it, is initially empty. The valve is opened and...
-
Solve the equation (a) Graphically, (b) Numerically, and (c) Symbolically. Then solve the related inequality. |4x7| = 5, |4x - 7| 5
-
Use the test scores of 24 students taking Marketing 235 to complete the frequency distribution and find the grouped mean rounded to the nearest whole number: 57 91 76 89 82 59 72 88 76 84 67 59 77 66...
-
In a certain process 2.15 X 105 J of heat is liberated by a system, and at the same time the system contracts under a constant external pressure of 9.50 X 105 Pa. The internal energy of the system is...
-
Recognize the various terms that pertain to products and services.
-
Rams Ramachandran is considering the wisdom of reducing the number of suppliers his firm uses. Currently, Rams uses 25 suppliers to purchase goods worth $2,500,000 per year. To manage the orders and...
-
(Holding-period dollar gain and return) Suppose you purchased 14 shares of Disney stock for $ 28.52 per share on May 1, 2009. On September 1 of the same year, you sold 14 shares of the stock for $...
-
Logan B. Taylor is a widower whose wife, Sara, died on June 6, 2016. He lives at 4680 Dogwood Lane, Springfield, MO 65801. He is employed as a paralegal by a local law firm. For 2018, he reported die...
-
A 2-kg piston accelerates to 20 m/s from rest. What constant gas pressure is required if the area is 10 cm 2 , the travel is 10 cm, and the outside pressure is 100 kPa?
-
A piston/cylinder assembly contains 2 kg of liquid water at 20C and 300 kPa, as shown in Fig. P3.48. There is a linear spring mounted on the piston such that when the water is heated, the pressure...
-
Let (x) = 20 + x x 2 and g(x) = x 2 5x. Sketch the region enclosed by the graphs of f and g, and compute its area.
-
Shamika conducts a study comparing the performance of individuals on the first quiz in each of two classes taken in the first term of their freshman year. The scores, out of 100, are provided for...
-
Adult Sleep Times (hours) of sleep for randomly selected adult subjects included in the National Health and Nutrition Examination Study are listed below. Here are the statistics for this sample: n =...
-
Topic: I made a couple changes X Zag HCHW Ch 2 (31) (1).pdf X W2ag HW Ch 1 (20) - Physics 2ag X /Desktop%20user/Downloads/2ag%20HCHW%20Ch%202%20(31) %20(1).pdf f o search Hi 2/14 - I + 90% + 1.1 a....
-
Cashless Society. 40% of consumers believe that cash will be obsolete in the next 20 years (based on a survey by J.P. Morgan Chase). In each of Exercises 15-20, assume that 8 consumers are randomly...
-
Pacifico Company, a U . S . - based importer of beer and wine, purchased 1 , 3 0 0 cases of Oktoberfest - style beer from a German supplier for 3 1 2 , 0 0 0 euros. Relevant U . S . dollar exchange...
-
What are the greatest inherent risks in the purchasing process? Explain the assertions that are at risk and the underlying drivers causing an increase in inherent risk.
-
The financial statements of Eastern Platinum Limited (Eastplats) are presented in Appendix A at the end of this textbook. Instructions (a) Does East plats report any investments on its statement of...
-
Assume a setup similar to the previous problem but the air flows in the opposite direction of the glass, it comes in where the glass goes out. How much air flow at 17oC is required to cool the glass...
-
Three air flows all at 200 kPa are connected to the same exit duct and mix without external heat transfer. Flow one has 1 kg/s at 400 K, flow two has 3 kg/s at 290 K and flow three has 2 kg/s at 700...
-
Consider the power plant as described in Problem 6.102. a. Determine the temperature of the water leaving the intermediate pressure heater, T13, assuming no heat transfer to the surroundings. b....
-
1. Why might managers of small restaurants decide not to adopt the standard work hour approach to controlling labour cost? (minimum 150 words )
-
Which statement is true regarding the U.S. GAAP impairment test for limited life intangibles? A. U.S. GAAP impairment is likely to be greater than IFRS impairment. B. The impairment test for limited...
-
Which of the following is a limitation of both return on investment and residual income? A. Favors large units. B. There is disincentive for high return on investment units to invest. C. Can lead to...

Study smarter with the SolutionInn App


